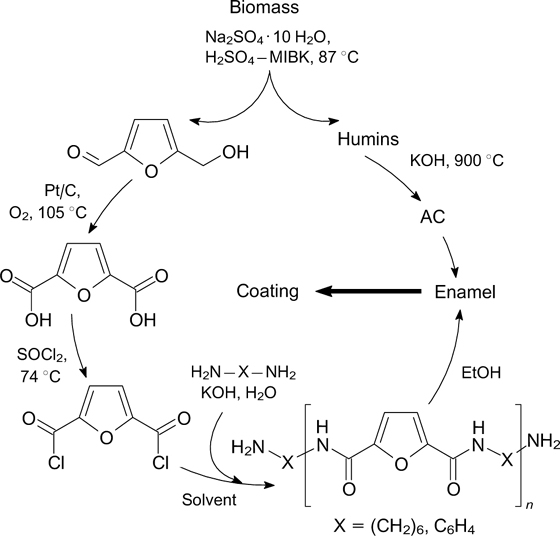
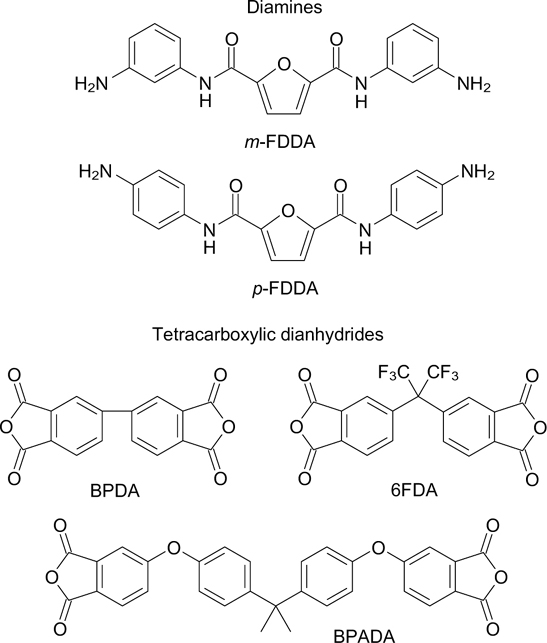
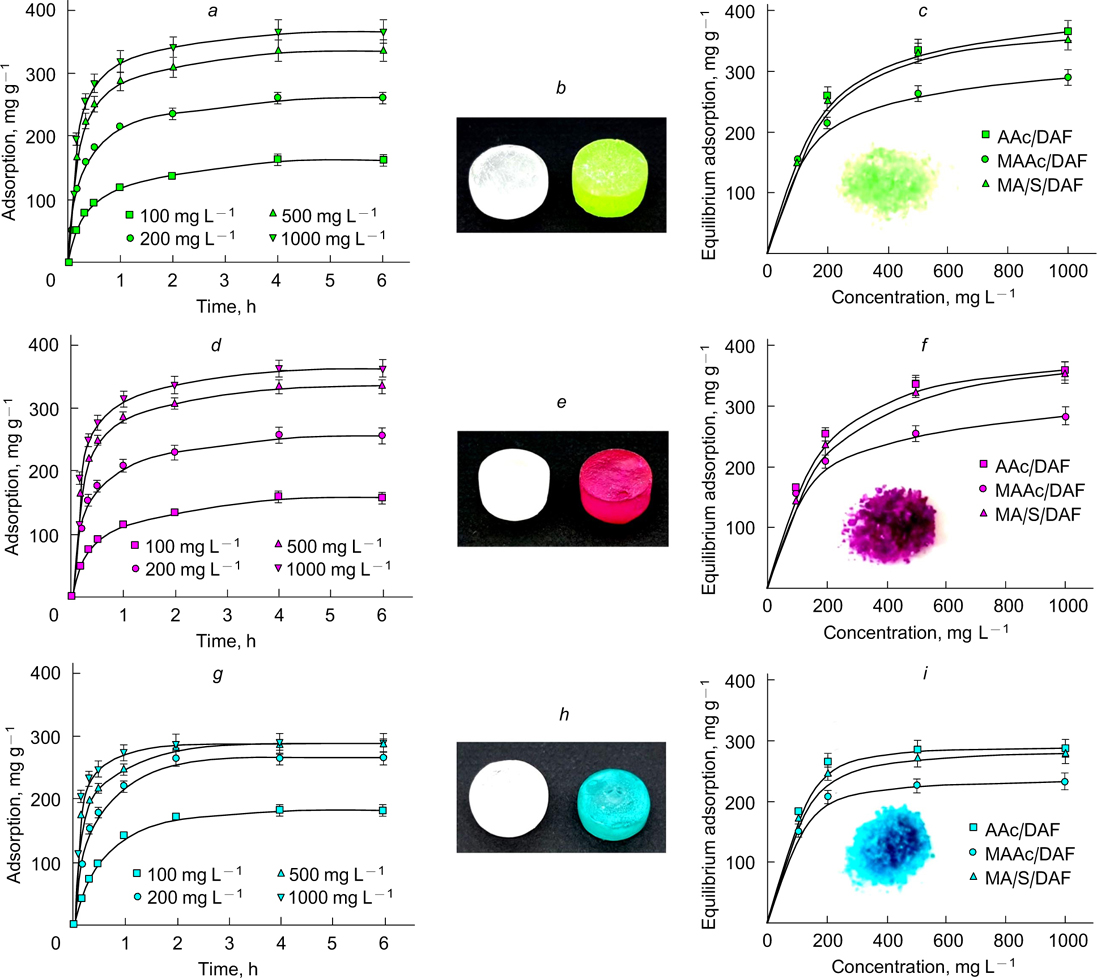
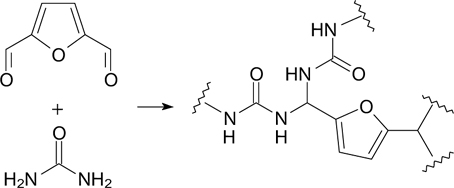
Abstract
Plant biomass is considered the main source of renewable carbon raw materials, which is a viable alternative to crude oil and natural gas and provides compounds with a low carbon footprint. The most promising direction for the conversion of biomass is the synthesis of 5-hydroxymethylfurfural, which is regarded as a platform chemical, the basis for the synthesis of valuable compounds, including monomers and polymers. The move of the polymer industry to renewable plant materials will contribute to solving global environmental problems and ensure the sustainability and environmental safety of plastics production. This review analyzes recent advances in the preparation of key C6-furan platform chemicals, such as 5-hydroxymethylfurfural, 2,5-furandicarboxylic acid, 2,5-diformylfuran, 2,5-bis(hydroxymethyl)furan, levulinic acid, and their use for the production of monomers and polymers based on renewable plant biomass. Production processes of widely known furan polyesters and polyamides, such as polyethylene-, polypropylene-, polybutylene furanoates, polyhexamethylene furanamide, are considered, as well as developments towards novel promising materials that are furan copolymers and polymer mixtures. For the first time, studies have been systematized aimed at converting liquid and solid wastes of the synthesis of platform chemicals, known as humins, into a range of value-added products, including organic compounds, porous carbon materials, thermosetting binders, and anticorrosive coatings that can replace similar materials based on fossil raw materials. Economic and environmental prospects and problems of production and consumption of furan polymers are considered.
The bibliography includes 275 references.
Export citation and abstract BibTeX RIS
| V.P.Kashparova. PhD in Technical Sciences, Associate Professor at the Department of Chemical Technologies of the SRSPU (NPI). |
| E-mail: kashparova2013@mail.ru |
| Current research interests: electrochemistry of organic compounds, polymer chemistry. |
| D.V.Chernysheva. PhD in Technical Sciences, Senior Researcher at the Research Institute 'Nanotechnology and New Materials' of the SRSPU (NPI). |
| E-mail: da.leontyva@mail.ru |
| Current research interests: electrochemistry of organic compounds, composite and hybrid materials for heterogeneous and electrocatalysis. |
| V.A.Klushin. PhD in Technical Sciences, Associate Professor at the Department of Chemical Technologies of the SRSPU (NPI). |
| E-mail: victorxtf@yandex.ru |
| Current research interests: chemical processing of plant raw materials, polymer chemistry, electrochemistry. |
| V.E.Andreeva. PhD in Technical Sciences, Researcher at the Research Institute 'Nanotechnology and New Materials' of the SRSPU (NPI). |
| E-mail: veronica.andreeva@gmail.com |
| Current research interests: chemistry of polymers, biotechnology, adsorption of heavy metal cations. |
| O.A.Kravchenko. Doctor of Technical Sciences, Associate Professor, Acting Rector of the TSU. |
| E-mail: mvk346428@gmail.com |
| Current research interests: catalysis, chemical processing of plant raw materials, the chemistry of sustainable development. |
| N.V.Smirnova. Doctor of Chemical Sciences, Professor at the Department of Chemical Technologies of the SRSPU (NPI). |
| E-mail: smirnova_nv@mail.ru |
| Current research interests: electrochemistry of organic compounds, composite and hybrid materials for heterogeneous, electro- and photocatalysis. |
1. Introduction
The economy of modern society is based on the use of non-renewable fossil resources (oil, gas, coal), which currently provide ∼80% of energy and ∼90% of the carbonaceous feedstock for the chemical industry. 1,2 The depletion of fossil hydrocarbon reserves stimulated the search for new sources of energy and raw materials based on renewable resources. Plant biomass is considered the main source of renewable carbon raw materials, which is a real alternative to crude oil and natural gas and provides compounds with a low carbon footprint. 3–5 The most promising trend in the biomass processing is the dehydration of carbohydrates to give 5-hydroxymethylfurfural (HMF), which, in turn, is considered as a 'platform molecule' for the synthesis of a variety of valuable substances and materials, including monomers and polymers, and the sustainable development of the chemical industry. 2,6–8
This review focuses on the analysis of the latest developments in the preparation of key furan compounds such as HMF, 1–4,8 2,5-furandicarboxylic acid (FDCA), 2,9–13 2,5-diformylfuran (DFF), 9,10,14,15 2,5-bis(hydroxymethyl)-furan (BHMF), 16–18 levulinic acid (LA), 2,10,19,20 and their use to obtain furan polymers from renewable plant biomass. An assessment of all possible HMF derivatives described in the literature is beyond the scope of this review since the number of potential HMF derivatives is enormous. The review provides information on recent advances in the utilization of typical and new catalytic biomass conversion systems; general mechanisms of synthesis of furan compounds are discussed; novel furan monomers and polymers are described; emphasis is placed on works devoted to new environmentally friendly and low-waste processes. The bulk of the cited literature represents the developments and technological advances in this area over the past three years. Earlier works were used for a comparative and more comprehensive description of the challenges posed by the synthesis of furan platform chemicals, as well as monomers and polymers derived therefrom. Mention has been made of the contribution of Russian researchers to the solution of the problems of renewable plant biomass processing into valuable furan compounds and polymers.
Achievements in the synthesis of key furan compounds using various carbohydrate raw materials, homogeneous and heterogeneous catalytic systems, traditional and innovative approaches to the choice of technologies for their use are summarized in Section 2. Various ways of converting HMF into valuable chemical products through oxidation, hydrodeoxygenation, esterification, amination, hydrogenation, and cycloaddition are analyzed. The advances and existing difficulties in the process of converting glucose, cellulose, and other polysaccharides into HMF, FDCA, DFF, and BHMF are noted.
Approaches and methods for obtaining polymers based on furan platform chemicals and their derivatives are discussed and analyzed in Section 3. Opportunities and prospects are considered, and the importance of replacing polymers based on fossil raw materials with similar materials based on plant biomass is noted. The transition of polymer industry to bio-renewable materials would certainly contribute to combating the global environmental issues which are associated with the petrochemical and petroleum sectors, on which the current polymer industry is heavily dependent, and this would undoubtedly ensure the sustainability and environmental safety of the polymer industry. 21–23 The results of studies of various monomers such as FDCA, FDME, DFF, BHMF, and LA, reaction conditions, initiators and catalysts, and also structural and thermomechanical properties of the produced polymers are presented. Attention is paid both to improving production processes for the well-known furan polyesters and polyamides such as poly(ethylene furanoate) (PEF), poly(propylene furanoate) (PPF), poly(butylene furanoate) (PBF), 24 poly(hexamethylene furanamide) (PA6F), 25 and developing novel promising copolymers and polymer blends. Studies on production and practical use of novel copolymers based on unsaturated esters of furandicarboxylic acids are first discussed. 26
As a promising trend in synthetic and polymer chemistry, biocatalytic polymerization of furan compounds opening access to the corresponding polyesters and polyamides without the use of toxic metal-containing catalysts under mild conditions is considered.
The development of completely zero-waste methods of synthesis, especially based on raw materials of plant origin, is difficult to implement, since the synthesis of furan platform chemicals gives a large amount of liquid and solid wastes, known as humins. 27,28 In Section 4 of the present review, we first attempted to summarize the data published to date concerning the possibility to convert humins into a wide range of value-added products. Among possible products, lactones, phenols and organic acids, 29,30 porous activated carbon materials used as sorbents, 31–33 electrode materials for electrochemical power devices, 34 and heterogeneous catalysts 35,36 are described. Special attention is paid to the possibility of practical application and preparation of the humin-based various multicomponent thermosetting construction materials, 37–45 binders to increase the strength of wood and improve the adhesion in the manufacture of green composites (plywood, wood fibreboard), 46,47 anticorrosive coatings, 48,49 which can replace analogous materials and coatings based on fossil fuels.
Economical and environmental aspects of production and consumption of polymers, primarily, plastic packaging, which currently leads to excessive emissions of carbon dioxide into the environment, are discussed in Section 5. Special attention is paid to the economic assessment of prospects for the use of renewable plant biomass for the production of furan platform chemicals and polymers derived therefrom. Replacing traditional plastics with biomass-based alternative materials will facilitate the transition to a low-carbon economy with minimal CO2 emissions, which is important for mitigating the effects of climate change and preserving the Earth's natural resources. 21,50–52
The authors hope that the review will serve as a useful reference material for researchers specializing in the development and synthesis of new furan monomers and polymers from renewable plant biomass, and will draw the attention of industry representatives to the most important scientific and technological problems, the solution of which will help to overcome the current challenges in this area.
2. Synthesis of furan platform chemicals, including monomers, from the renewable plant biomass
2.1. Synthesis of 5-hydroxymethylfurfural from fructose
The most important area of biomass processing is the production of HMF and its valuable derivatives (Fig. 1). 2,4,53–57
Figure 1. Possible pathways for converting biomass into HMF and its derivatives. 2 Reprinted with the permission of the Royal Society of Chemistry.
Download figure:
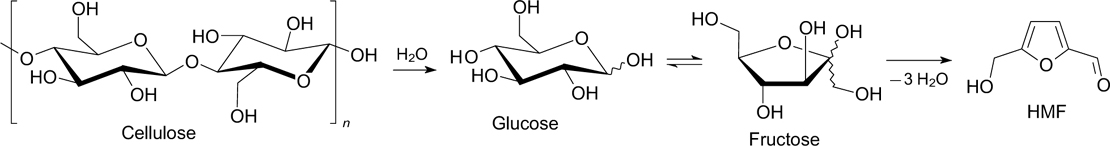
Standard imageThe main intermediate in biomass conversion to HMF is fructose. In this case, fructose can be used both in pure and crude forms, when the multistep HMF synthesis is carried out as a one-pot process. 2
One of the plausible pathways for the formation of HMF from fructose through cyclic intermediates is illustrated in Scheme 1. 1
Scheme 1
Download figure:
Analysis of the literature data on the mechanisms and kinetics of dehydration of fructose and other hexose carbohydrates to HMF is detailed in a review. 53 Researchers are considering the conversion of fructose to HMF under both homogeneous and heterogeneous catalysis. 2 In turn, homogeneously catalyzed reactions are carried out in organic solvents, aqueous media, and ionic liquids (ILs). 1,58 Certain organic solvents, e.g., dimethyl sulfoxide (DMSO), are widely used as reaction media in the synthesis of HMF. Despite the declared high yields of HMF (up to 98%), there have been challenges in isolating the target product and regenerating the solvent. 59,60 The prospects of using ILs as media for the synthesis of HMF stem from the good solubility of cellulosic biomass in them and the possibility of eliminating side reactions (isomerization, dehydration, fragmentation, and polymerization) that occur in common organic solvents. 60,61 However, the laborious procedure for isolating HMF and the high cost of the ILs themselves limit the scope of these methods for HMF synthesis outside research laboratories. Therefore, despite all the advantages of syntheses in organic solvents or ILs using various catalysts, laboratory 62 and industrial 63 processes for the production of HMF are carried out using homogeneous catalysis in aqueous and two-phase media.
Note that HMF is unstable in aqueous solutions, which is associated with a special arrangement of molecules caused by a hydrogen-bond network, which was detected using spectral methods (NMR and ESI-MS). This behaviour of HMF promotes its rapid degradation. 64 The lack of stability in an aqueous medium and during storage of an insufficiently purified product, as well as the occurrence of side reactions during the isolation and further modification of HMF, preclude its preparation in a high yield and limit the use of this most important bio-renewable platform chemical in organic synthesis. 65 To circumvent this issue and increase the yield of the target product, two-phase aqueous-organic systems are used, which provide the extraction of HMF from the reaction zone with organic solvents. 58,62,66
Currently, the application of heterogeneous catalysis in the synthesis of HMF is of increasing interest. 2 Heterogeneous catalysis has many advantages, such as ease of product isolation from the reaction mixture and catalyst regeneration. However, there are also several drawbacks, namely, the dependence of the reaction rate on the catalyst surface area, the need for a large amount of catalyst, rapid catalyst deactivation during use, the need for higher temperatures than with homogeneous catalysis, which entails high energy costs in the production of HMF. Therefore, the efforts of researchers are aimed at developing new and optimizing existing heterogeneous catalytic systems.
As heterogeneous catalysts, solid acids such as polymers containing sulfonic acid groups (–HSO3), 67 modified carbon materials, 68,69 and inorganic oxides 70–73 are used. Sonsiam et al. 67 investigated the process of fructose dehydration using a cation-exchange resin functionalized with sulfonic acid groups. To enhance the product yield, water-organic mixtures with methyl isobutyl ketone and N-methylpyrrolidone (NMP) were used. The highest yield of HMF (91.45%) was attained at a temperature of 120 °C, a reaction time of 30 min, and an NMP : water ratio of 7 : 3. Dai et al. 74 reported the application of sulfonated polyaniline as a catalyst (71% yield of HMF).
The use of activated carbon fails to provide high yields of HMF (∼15%), but at the same time, concentrated aqueous solutions containing up to 73% fructose can be used as raw materials. 68 Catalysis with sulfonated carbon improves the yields of HMF up to 75%, and the use of a DMSO – H2O (10 : 1) mixture as a solvent in place of water helps to achieve 94% yield of HMF. 69
The use of titania modified with chlorosulfonic acid as a catalyst at 165 °C provided HMF in 50% yield and complete conversion of fructose (1.1 M solution). 71 With a decrease in temperature (140 °C) and fructose concentration (0.1 M), the yield increased to 71%. The application of silica modified with sulfonated fluoropolymer 72 ledto85% yield of HMF even at a temperature of 90 °C. Alumina treated with cetyltriammonium bromide 73 affords HMF in 51% yield at 200 °C in 5 min.
2.2. Synthesis of 5-hydroxymethylfurfural from glucose
It has been established that exactly fructose is the precursor of HMF. To convert glucose into HMF, the first step should include its isomerization to fructose. 75 Isomerization proceeds in an alkaline medium; therefore, acidic catalysts used in the synthesis of HMF from glucose do not provide high yields. Currently, catalysts are being investigated, combining acidic sites for fructose dehydration and basic sites for glucose isomerization. Thus, the mechanism of glucose isomerization under catalysis with sulfanilic acid has been studied. 75 Subsequent dehydration of fructose over this catalyst gave a 44% yield of HMF.
However, it should be noted that glucose can be isomerized to fructose also in an acidic medium, albeit very slowly. Therefore, the synthesis of HMF from glucose proceeds at high temperatures with the use of two-phase systems containing extracting agents. Li et al. 76 carried out the synthesis using hydrochloric acid as a catalyst and γ-valerolactone (GVL) as an extracting agent at 140 °C, having achieved 62% yield of the target product (Fig. 2).
Figure 2. One of the possible ways for producing HMF in an H2O/GVL – HCl system. 76 Reprinted with the permission of the Royal Society of Chemistry.
Download figure:
Standard imageTo obtain HMF from glucose, IL is used rather often. Thus, Phan et al. 77 reported the synthesis of HMF from monosaccharides using a novel binary IL as a reaction medium. The Brønsted – Lewis acidic ionic liquid obtained from 1,4-diazabicyclo[2.2.2]octane (DABCO), 1,4-butane sultone, and AlCl3, was employed as a catalyst, and [Emim]Cl was used as a solvent. Under optimized conditions, the yields of HMF from glucose and fructose were 30.5 and 96.5%, respectively. It was found that the mixture of ionic liquids can be reused three times without a noticeable loss in catalytic activity.
Recently, the bulk of research on the production of HMF from glucose has been devoted to the development and study of heterogeneous catalysts. 78–84 For instance, carbon materials treated with IL or doped with nitrogen are used. Matsagar et al. 79 succeeded to obtain HMF from glucose in a 39% yield under catalysis with bone charcoal treated with 1-methyl-3-(3-sulfopropyl)imidazolium hydrogen sulfate. Zhang et al. 80 used nitrogen-doped carbon nanotubes as a catalyst, and the yield of HMF in a DMSO medium was 63 %. Metal oxide catalysts combining acidic and basic sites are being developed and applied. Thus, the use of Al2O3 –TiO2 – W catalysts affords HMF in a 70% yield; 81 L-type zeolite provides a 63% yield at 175 °C, 82 while HY and HMOR zeolites treated with 1-butyl-3-methylimidazolium bromide give a 39 % yield at 170 °C. 83
Yin et al. 85 reported the synthesis of HMF from glucose in IL using MoCl3 as a catalyst under microwave irradiation. The results demonstrated that compared to conventional heating in an oil bath, microwave irradiation not only shortens the required reaction time from hours to minutes but also improves the yield of HMF. With a catalyst loading of 10 mol.%, the yield of HMF reached ∼21% after keeping the reaction mixture for 2.5 h at 120 °C inanoil bath, while under 300 W microwave irradiation, a comparable yield of HMF (∼28%) was attained in 5 min. Coordination bonds formed between MoCl3 – [Bmim]Cl complex and glucose promotes its isomerization to fructose and dehydration of the latter to HMF.
Despite the significant research interest in the production of HMF from glucose, no information on industrial or scalable laboratory production methods is available.
2.3. Synthesis of 5-hydroxymethylfurfural from renewable inedible feedstock (cellulose and other polysaccharides)
Industrial production of HMF from sugars (glucose, fructose, sucrose) is irrational since it consumes, rather expensive food resources necessary for mankind. Therefore, developments aimed at using cellulose as a feedstock are of increasing interest. 2 Cellulose is the most abundant polysaccharide, as plant biomass is mainly composed of lignocellulose. In several sectors of the economy, a huge amount of cellulose-containing waste is generated, for example, in agriculture, ranging from straw to pulps remaining after extracting juices from plants; in the wood-processing industry, chips and sawdust are formed as waste. 86
It is noteworthy that the main drawback of cellulose as a feedstock is its insolubility in water and organic solvents. Therefore, the synthesis of HMF from cellulose is usually a multistage procedure. In the first stage, the cellulosic biomass is hydrolyzed to glucose, which is subsequently isomerized to fructose and dehydrated to HMF (Scheme 2). 1
Scheme 2
Download figure:
To achieve better solubility of cellulose, the process is usually carried out in ionic liquids. Galkin et al. 87 used 1-butyl-3-methylimidazolium chloride as a solvent and chromium(III) chloride as a catalyst, with the yield of HMF being 52%. In the acidic IL [1-hexyl-3-methylimidazolium hydrogen sulfate ([Hmim]HSO4)], the yields of HMF obtained from D-glucose and D-fructose were similar (76.1% and 77.3%, respectively), while starting from cellulose, the yield of HMF was 68% irrespective of the molecular weight and crystallinity of the substrate. 88
While ILs are attractive, their cost does not yet allow the application of the aforementioned methods on an industrial scale. Surveys are ongoing on the search and study of aqueous and water-organic systems. Gromov et al. 89 implemented the hydrothermal method with the use of metal oxide NbOx –ZrO2 catalysts to obtain HMF in 16% yield. The application of a graphite-like mesoporous material Sibunit helped to improve the yield of HMF to 22%. 90 In a follow-up study, 91 the mechanism of cellulose depolymerization in the presence of the same catalytic system was carefully examined. Zirconium-doped mesoporous silicas 92 used as catalysts were active in converting cellulose to HMF in 45% yield. Florez-Velázquez et al. 93 studied the processing of banana peel into HMF in the presence of Al2O3 – TiO2 – W and the relationship between the degree of grinding of raw materials and the yield of the target product. The use of ZSM-5 zeolite 94 in dehydration of the residual biomass of algae in a two-phase system tetrahydrofuran – water provides 34% yield of HMF.
The company AVA Biochem has developed a technology for large-scale production of HMF by hydrothermal processing of biomass 63,95 and planned in 2020 – 2022 the construction of a plant for producing HMF with an annual capacity of 5 – 6 thousand tons.
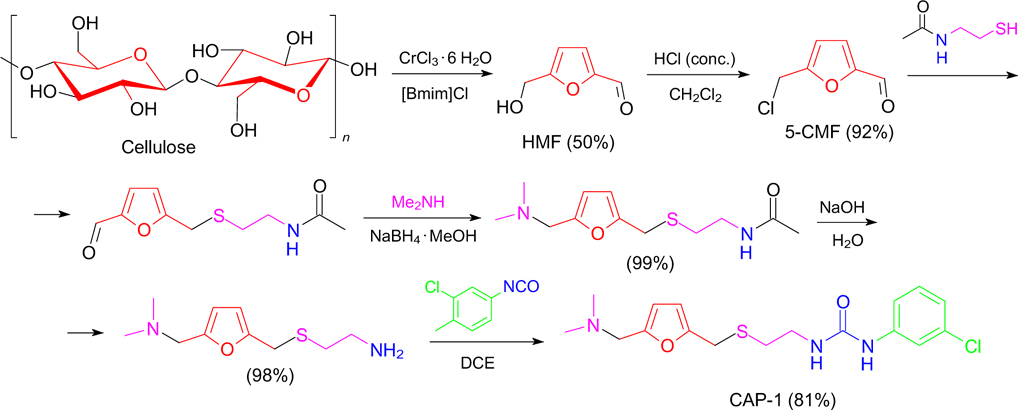
Along with the need to develop advanced methods for the synthesis of HMF, its practical use is no less important. In recent years, intensive researches have been underway to obtain HMF-derived biofuels, various chemicals, pharmaceuticals, monomers and polymers, as evidenced by a huge number of publications. 1–4,96 In particular, Romashov and Ananikov 97 synthesized structural analogues of N-(3-chloro-4-methylphenyl)-N'-2-[(5-[(dimethylamino)methyl]-2-furylmethyl)sulfanyl]ethylurea (CAP-1) based on cellulose-derived HMF (Scheme 3). The resulting preparations show anti-HIV activity and offer significant opportunities for the synthesis of pharmaceuticals starting from biomass.
Scheme 3
Download figure:
Another important area of HMF application is the synthesis of monomers for the production of biopolymers. 96 Dimethyl furanoate (FDME), 98 which is one of the monomers for PEF synthesis, was obtained by a one-pot procedure from HMF. Oxidative esterification of biomass-derived HMF and furfural was carried out using a simple system MnO2 – NaCN. This method provides the selective one-pot conversion of HMF to FDME in 83% yield without the formation of free FDCA.
The high cost of fructose, which is currently employed for low-tonnage production of HMF and the relatively low efficiency of the proposed methods for obtaining HMF from cheap cellulose, are still the main obstacles to its production on an industrial scale and application in organic synthesis, including that of monomers such as FDCA. The development of technologies for obtaining HMF from available feedstock, such as plant biomass, faces the need to use toxic and(or) expensive media (e.g., IL), in the case of homogeneous catalysis, or the energy-consuming process of biomass dehydration, when using heterogeneous catalysis. It should be noted that both of these approaches do not yet provide high yields of HMF. Therefore, the need arises for involving alternative energy sources, for example, solar or hydrothermal, together with the improvement of heterogeneous catalysis protocols (the optimal combination of an effective catalyst and solvent) for converting biomass into HMF.
2.4. Synthesis of 2,5-furandicarboxylic acid and its derivatives
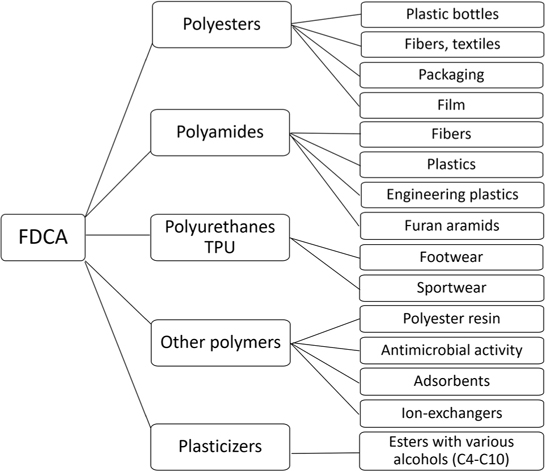
2,5-Furandicarboxylic acid (FDCA) is a platform chemical 11–13,63 and represents an alternative to the so-called petroleum acids, namely, terephthalic and isophthalic acids, being capable of replacing them in the production of plasticizers, monomers, and various polymers based on renewable plant biomass 24,26,99–103 (Fig. 3).
Figure 3. Possible areas of processing FDCA into various polymers and materials. Adapted from the data of Refs 11 and 63.
Download figure:
Standard imageAmong the main research areas in the synthesis of FDCA, heterogeneous catalysis, 66,95,104–111 electrocatalysis, 9,112–116 photocatalysis, 9,117,118 photoelectrocatalysis, 114,119–121 oxidation with inorganic stoichiometric oxidants, 12,122,123 and biocatalysis 124–126 can be highlighted. Each of the above-mentioned approaches has own advantages but not without drawbacks.
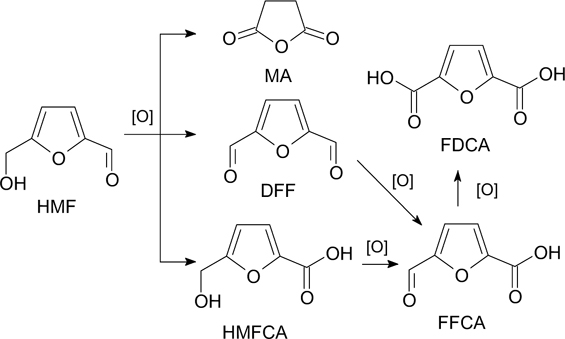
Heterogeneous catalytic oxidation of HMF with air, molecular oxygen, or peroxides is considered the most promising for implementation on an industrial scale. 1,95,104,105 During the oxidation process, various intermediates and by-products can be formed (Scheme 4). 1
Scheme 4
Download figure:
Among the numerous studies 66,95,104–111 devoted to the production of FDCA under heterogeneous catalysis, three main types of catalysts can be distinguished: (i) noble metal catalysts (Pt, Au, Pd, Ru), 13,95,106–110 (ii) bimetallic catalysts, (PdAu, PdBi, AuCu, etc.), 13,95,110 and (iii) metal oxide catalysts (NiOx , MnOx , CoOx , ZnOx , FeOx , CeOx ). 13,66,95,111 The main advantage of such catalytic systems is their high activity, stability and, which is important in terms of industrial implementation, the possibility of multiple recycling, as noted in most works related to heterogeneous catalysis. 13,95 Activity and selectivity of heterogeneous catalysts are influenced not only by the composition, crystallographic orientation, and microstructure of metal nanoparticles but also by the nature and composition of the support (various forms of carbon, zeolites, metal oxides). 12,95,106,110 Thus, German et al. 110 investigated the effect of the support (unmodified and modified TiO2, aluminium-based supports, untreated and treated Sibunit carbon) of mono- and bimetallic catalysts based on noble metals (Ag, Au, Pd) on the selective oxidation of HMF with molecular oxygen [P(O2) = 0.3 MPa] to give FDCA in an alkaline medium. Higher selectivity to FDCA was obtained when metals were deposited on Sibunit. Selectivity of catalysts increased in the order: Ag < Au < Pd < PdAu. Functionalization of Sibunit surface with HNO3 and NH4OH alters the oxidation states of Pd and Au due to the promoting effect of N-doping and, as a consequence, increases the yield of FDCA up to 76%.
Despite the possibility to obtain FDCA in a high yield, one of the major drawbacks of the proposed protocols of heterogeneous catalytic oxidation of HMF to FDCA is the need to carry out the reaction under pressure and in an alkaline medium 13,95,110 to convert FDCA into the salt form, to avoid inactivation of the active sites of catalysts formed on their surface by poorly soluble FDCA, which leads to a significant amount of waste mineral salts and also increases the risk of reactor corrosion. 95
The formation of FDCA in a neutral (base-free) medium follows the pathway of oxidation of the hydroxyl group with the formation of the intermediate DFF, which is further oxidized to FFCA (see Scheme 4). Oxidation of HMF with the so-called base-free catalytic systems is more environmentally friendly and economically viable due to the absence of additional steps of neutralization and separation of the final product. However, despite the obvious advantages, the proportion of base-free systems among those described in the literature is small, 107,108,111 which is associated with the above-mentioned problem of possible inactivation of the catalyst by the target product, which causes relatively low average yields of FDCA. For instance, Chernysheva et al. 106 proposed a method for selective aerobic [0.25 MPa (O2), 105 °C] oxidation of concentrated aqueous solutions of HMF (0.5 mol L−1) with an FDCA yield of up to 65% in the absence of bases over Pt/C catalysts obtained by electrochemical dispersion under pulsed alternating current. Relatively high platinum content (∼30%) in the developed catalysts prevents side reactions of the humic substance formation.
Along with noble metal-based catalysts, 106,107 oxide catalytic systems are also being investigated. Gao et al. 111 succeeded to obtain FDCA in a yield of above 99% when carrying out the synthesis within 24 h at 140 °C, 0.1 MPa (O2), with the use of Co3O4/Mn0.2Co catalyst, which has both Lewis (Mn4+) and Brønsted (Co–O–H) acid active sites.
In respect of the prospects for the development of industrial production processes of FDCA, it is obvious that one-pot two-step methods of converting carbohydrate feedstock (for example, fructose 108,127 ) into FDCA without the step of HMF isolation deserve special attention as not requiring purification, storage and transportation of unstable HMF. One of the few works in this field is the preparation of FDCA in 93% yield from fructose in a mixture of γ-valerolactone (GVL) and H2O in which FDCA served as an acidic catalyst for the dehydration of fructose, and Pt/C was used a catalyst for the oxidation of HMF to FDCA. 108 This study is noteworthy because of the proposed approach to the choice of a catalyst and solvent which can be obtained during the process (FDCA) or from by-products (GVL from LA).
Electrochemical oxidation (ECO) of HMF is a promising, environmentally friendly, and waste-free way to obtain FDCA. 9,112–116 High selectivity and rate of electrocatalysis can be achieved by varying the applied potential, current density, and catalyst type. In ECO, the electric current acts as an oxidant, and in aqueous electrolytes, hydrogen and oxygen are the main by-products. On a nickel oxide electrode, alcohols and aldehydes are electrochemically oxidized to acids in 100% yield, which is the reason for plenty of works devoted to the use of various nickel-containing catalysts for the selective production of FDCA. 112,115,116 In 0.1 M KOH solution on a NiOOH electrode in a potentiostatic mode at 1.47 V (versus RHE), FDCA is formed in 96% yield, while CoOOH and FeOOH electrodes are not active in this reaction. 112 Electrooxidation of HMF using S–Ni@C 115 and Ni3N@C catalysts 116 provides FDCA in yields of 96 and 98%, respectively. In this case, the 98% yield was retained after six catalytic cycles on the Ni3N@C 116 electrode, indicating the prospects for the industrial implementation of electrocatalytic processes in general.
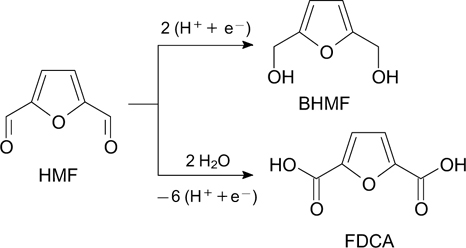
In addition to direct electrooxidation of HMF, the possibilities of using mediator systems for the synthesis of FDCA are being investigated. Application of an electrochemical cell with cathode and anode chambers separated with an anion-exchange membrane gave two important compounds, BHMF and FDCA (85 and 98%, respectively), at once in a high yield (Scheme 5). 128 As a cathode for electrocatalytic hydrogenation of HMF to BHMF, Ag/C was used, and on a glassy carbon anode, an indirect electrooxidation of HMF catalyzed by TEMPO (2,2,6,6-tetramethylpiperidine 1-oxyl) occurred.
Scheme 5
Download figure:
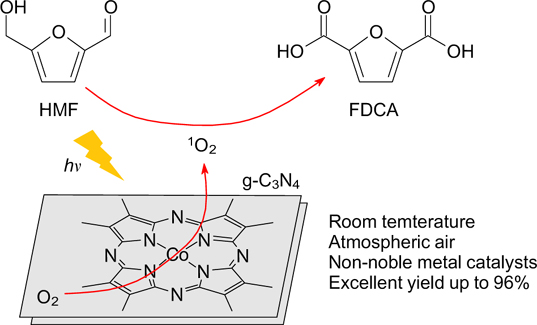
Photocatalytic transformations of organic compounds are becoming increasingly popular due to the simplicity and the possibility of using atmospheric oxygen as an oxidant. Most of the photocatalytic oxidation systems of HMF described in the literature lead to the production of DFF as the main reaction product. 129–133 There are very few works on the selective photocatalytic synthesis of FDCA. In particular, Xu et al. 117 reported the use of a photocatalyst based on cobalt thioporphyrazine (CoPz) dispersed onto graphitic carbon nitride (CoPz/g-C3N4), which resulted in the formation of FDCA in 96.1% yield via selective oxidation of HMF in an aqueous solution at room temperature and atmospheric pressure with the possibility of recycling the photocatalyst (Fig. 4). The selectivity of this catalyst was shown to depend on the strictly defined pH value of the solution (pH 9.18), provided by the Na2B4O7 buffer.
Figure 4. Selective oxidation of HMF to FDCA with oxygen on a CoPz/g-C3N4 photocatalyst. 117 Reprinted with the permission of the American Chemical Society.
Download figure:
Standard imageGonzalez-Gasamachin et al. 118 selected a composite of zinc oxide and polypyrrole (ZnO/PPy) as a photocatalyst for HMF oxidation. According to HPLC, the main products obtained at 25 °C were HMFCA, FFCA, DFF, and FDCA with the selectivity of 55, 12.4, 0.4, and 30.4%, respectively. Thus, the search for photocatalytic systems capable of oxidizing HMF to FDCA selectively and in high yields is very relevant. 133
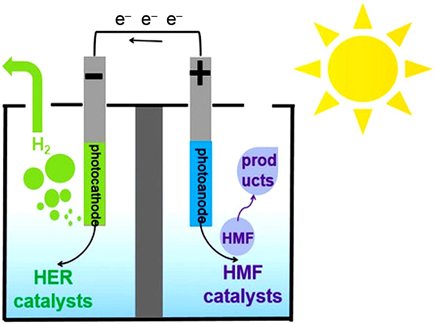
A relatively new and poorly studied area is the photoelectrocatalytic conversion of HMF. 114,119–121,133 In a standard photoelectrochemical cell, water is photooxidized on a photoanode, which provides the formation of electrons and protons used to evolve H2 at the cathode. Replacement of the slow water photooxidation with an alternative HMF oxidation (Fig. 5) can potentially increase the efficiency of converting solar energy into fuel (H2) by reducing the working anode potential. 121 An advantage of photoelectrodes is their ability to use sunlight directly to carry out photoelectrochemical reactions without applying an external voltage, which is beneficial from economic and environmental points of view. 114
Figure 5. Photoelectrochemical cell for HMF oxidation and parallel production of H2. 133 Reprinted with the permission of Wiley.
Download figure:
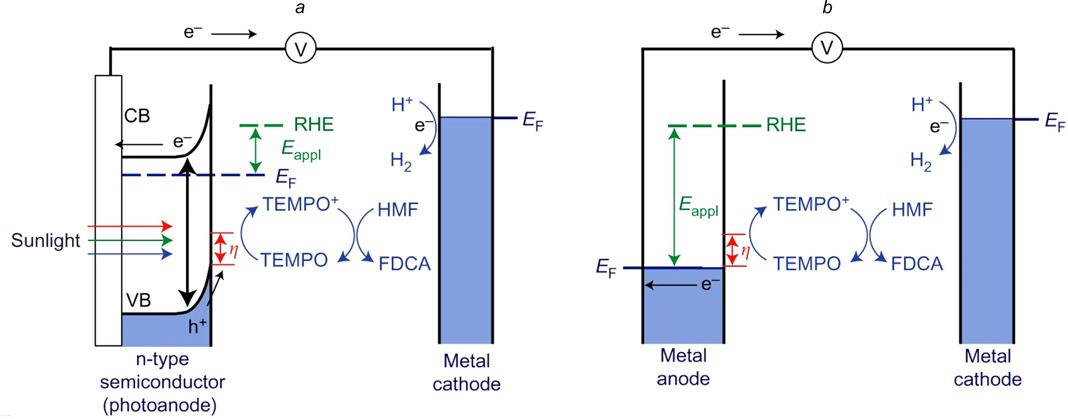
Standard imageCha and Choi 119 and Chadderdon et al. 120 found that the use of TEMPO as a mediator in a photoelectrochemical system with a BiVO4 photoanode significantly reduces the onset potential for HMF oxidation to FDCA by suppressing the water oxidation, which leads to the production of FDCA with a current efficiency of up to 93% (Fig. 6). 119
Figure 6. Comparison of the photoelectrochemical and electrochemical cells. (a) TEMPO-mediated photoelectrochemical oxidation of HMF to FDCA; (b) TEMPO-mediated electrochemical oxidation of HMF; CB is conduction band; EF is Fermi energy. 119 Reprinted with the permission of Nature Publishing Group.
Download figure:
Standard imageLhermitte et al. 121 were the first to demonstrate the possibility of direct photoelectrooxidation of HMF in an aqueous electrolyte (pH = 4) using a WO3-based photoanode, but they failed to achieve significant results (yield <1 %) and selective FDCA formation.
FDCA can be obtained via oxidation of HMF with so-called inorganic stoichiometric oxidizing agents, e.g., KMnO4. 12,122,123 A laboratory bench for the oxidation of furan compounds (HMF) has been developed and patented, 123 which was used to optimize the process by combining equipment for the synthesis and isolation of the final product in one unit. Boldyreva et al. 122 showed that the use of the laboratory bench 123 for the oxidation of raw HMF obtained from plant biomass with a basic substance content of ⩾ 60%, avoiding the costly and laborious steps of HMF purification, provides the formation of FDCA in a yield of at least 89% and a purity of at least 99%.
As an alternative to chemical methods for FDCA production, biocatalytic oxidation of HMF to FDCA by enzymatic catalysis (in vitro) and whole-cell catalysis (in vivo) is considered. 124–126 Although the use of enzymes and microorganisms makes this process more environmentally friendly and selective, and it proceeds under mild conditions, at the moment biotechnologies cannot yet compete with the chemical conversion of HMF when developing large-scale production due to insufficient knowledge, relatively high cost, and the need to use solutions with a low concentration of HMF, as well as a long process time (usually at least 24 h). 124
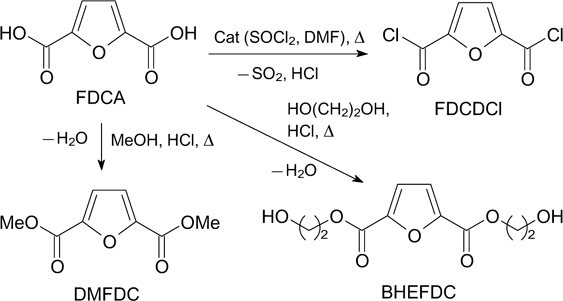
FDCA is an important synthon in the chemistry of furan compounds. It is used for the production of various biochemicals such as succinic acid, macrocyclic ligands, fungicides, furan polyesters and polyamides. 13,126 Moreover, such FDCA derivatives as dichloride (FDCDCl), dimethyl-(FDME), diethyl- or bis(hydroxyethyl)furandicarboxylate (BHEFDC) also represent monomers for the synthesis of polyesters, polyamides, and are base for plasticizers (Scheme 6). 13,134
Scheme 6
Download figure:
Recently, there has been a growing interest among various companies in the preparation of other FDCA-based monomers. Bisamides of FDCA are considered as curing agents for polyureas, hybrid epoxy-urethanes, hybrid urea – urethanes, and chain extenders for some elastomers, 135 and mixtures of FDCA diisodecyl ester are considered as plasticizers. 136
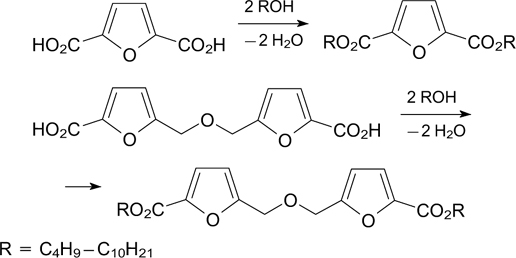
For the first time, the possibility of direct esterification of FDCA and 5,5'-oxybis(methylene)-bis-[2-furancarboxylic acid] (OBFA) with various aliphatic C4 –C10 diols was shown to give the corresponding diesters in a yield from 45 to 85% (Scheme 7). 137 Physical properties and plasticization ability of 14 obtained furandicarboxylic acid diesters were studied.
Scheme 7
Download figure:
However, the best-known and highly demanded FDCA ester is dimethylfuran-2,5-dicarboxylate (FDME), polycondensation of which with ethylene glycol affords a polyether PEF, intended to replace commonly used polyethylene terephthalate (PET). One-pot oxidative esterification of HMF to FDME is considered more cost-effective as requiring no isolation and purification of FDCA. 2,98,138–142
In most studies, cheap and easily available oxygen is used as an oxidant, thus forcing the synthesis to be carried out under pressure, 138–141 which complicates the instrumentation of the industrial production of FDME. The use of the noble metal catalysts 138,139 provides the synthesis of FDME in high yield (up to 96%), however, the proposed methods are still rather time-consuming (12 h, 140 20 h, 142 24 h 139 ) and costly. Preparation of FDME from fructose is the subject of not only fundamental research groups but also scientific departments of companies such as DuPont, Archer Daniels Midland, etc. 143,144
Despite the progress achieved in methods for converting HMF to FDCA, numerous attempts to develop a process that enables the production of FDCA and its derivatives in high yields on an industrial scale have so far failed. All currently known developments are at the stage of laboratory and pilot units, 11,13 using, inter alia, a process based on two catalytic reactions: (i) dehydration of carbohydrate feedstocks (C5 and C6 sugars) in an alcoholic medium to give alkoxymethylfurfurals and methyl levulinate and (ii) oxidation of alkoxymethylfurfurals in acetic acid to FDCA. 11
Given the above, to implement the industrial production of FDCA and its derivatives, the methods for obtaining FDCA should be developed and improved based on biomass carbohydrates (glucose, fructose, and cellulose), without isolating HMF, rather than on expensive purified HMF. However, the use of plant biomass as a substrate currently faces such problems as the complexity of the equipment, high energy consumption, and low yield of the target product. Also, it is necessary to optimize the existing methods for the synthesis of FDCA by increasing the activity and stability of the catalysts based on both noble and non-noble metals. It should be noted that electro-, photoelectro-, and metal-free catalysis are promising trends in FDCA preparation from economical and environmental points of view.
As for basic research, it is equally important to comprehensively study the mechanisms of the reactions of HMF oxidation to FDCA, an in-depth understanding of which will help in the development of new catalytic systems and the improvement of traditional synthetic approaches. Another important area is the study of the effect of various types of electromagnetic radiation and mechanochemical effects on the activity and efficiency of catalysts.
2.5. Synthesis of 2,5-diformylfuran and its derivatives
In addition to FDCA, one of the most valuable furan derivatives is another product of HMF oxidation, 2,5-diformylfuran (DFF). DFF is a platform chemical, 9,10,14,15,96 which can be used as a monomer to obtain urea – aldehyde resins, 145 polyimines, 146 electroconductive polymers, 147 porous polymer cages suitable for adsorption of CO2. 148 Moreover, DFF is a valuable building block for the preparation of various chemical compounds. 149 Unlike HMF, which is highly soluble in water, DFF is less hydrophilic and can be easily isolated from aqueous reaction mixtures by extraction with various organic solvents. 1,9,10,14,15,96,150 Selective conversion of HMF to DFF has been widely studied in the last 5 – 10 years. 9,10,14,15,61,96,129,131,132,150–159
Oxidation of HMF to DFF can be accomplished using stoichiometric oxidants such as potassium dichromate or chromium(IV) oxide, 160 sodium nitrite, 155 oxoammonium salts, 61 and also catalytic systems based on metals, oxides and salts. 156,157 Recently, organocatalytic systems have been in demand, both containing metals or metal salts 153,158,159,161 or not; 150–152 various photocatalytic systems are also gaining popularity. 129,131,132
Organocatalytic systems for oxidation of various compounds are generally characterized by high efficiency and ease of the product isolation. They include TEMPO-based catalytic systems. There are both chemical and electrochemical variants of such systems, the latter being considered environmentally and economically preferable. 9,150,162,163
In compliance with the principles of green chemistry, a method has been developed for the selective synthesis of DFF by indirect ECO of biomass-derived HMF under catalysis with inexpensive 4-acetylamino-2,2,6,6-tetramethylpiperidine-1-oxyl (4-AcNH-TEMPO) in a two-phase system consisting of methylene chloride and an aqueous solution of sodium bicarbonate and potassium iodide. 150 A key feature of this method is the formation of the I2 cooxidant through the anodic oxidation of iodide anions during pulse electrolysis (Fig. 7). It was shown that the electrolyte can be successfully used at least five times while maintaining the DFF isolated yield of 62 – 65%. This new approach provides a sustainable pathway for waste-free DFF production without the use of metal catalysts and expensive oxidants.
Figure 7. Scheme for 4-AcNH-TEMPO-mediated electrochemical oxidation of HMF to DFF. 150 Reprinted with the permission of Wiley.
Download figure:
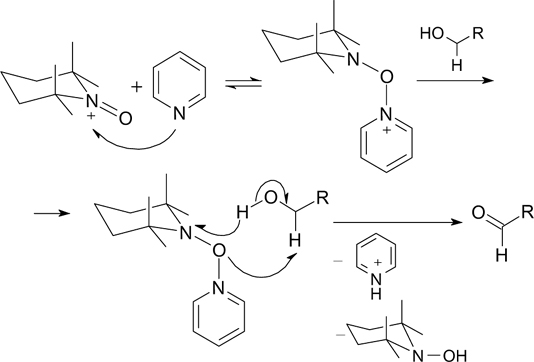
Standard imageTo improve the selectivity and the DFF yield, the effect of pyridine bases on the indirect ECO of HMF under catalysis with the 4-AcNH-TEMPO – KI system has been studied. 152 It was demonstrated that pyridine bases used in catalytic amounts (10 mol.%) promote the ECO reaction of HMF and other alcohols (the reaction is accelerated by 1.5 – 2 times). According to the proposed mechanism for the indirect ECO of alcohols in the presence of 4-AcNH-TEMPO and a pyridine base, the promoting effect of pyridine and other pyridine bases includes the formation of an intermediate complex between the base, the oxoammonium cation, and the substrate (Scheme 8). 152 The formation of this complex facilitates the rapid transfer of the hydroxyl proton to the pyridine base and the hydride ion to the oxoammonium cation with the conversion of the alcohol to the corresponding carbonyl compound. The yield of DFF was 85% at 100% conversion of HMF. The efficiency of the developed method was confirmed by the conversion of various alcohols into the corresponding carbonyl compounds in 75 – 95% yield.
Scheme 8
Download figure:
Chemical versions of the catalytic oxidation of alcohols, including HMF, which make use of TEMPO-based systems, are still popular, even though a similar system was proposed more than 30 years ago. 164
Ghatta et al. 153 studied the aerobic [P(O2) = 0.1 MPa] oxidation of HMF to DFF in the presence of TEMPO – CuCl catalytic system and bases in various imidazolium ionic liquids. The DFF yield of 90% is achieved with a catalyst loading of 5 mol% in 6 h at 80 °C and ahigh concentration of HMF (40%). Raising the temperature to 100 °C reduces the yield due to the release of volatile TEMPO from the reaction medium. A system that uses TEMPO and pyridine immobilized in an ionic liquid provides the selective conversion of HMF to high purity DFF, which can be recovered by sublimation in 81% yield before carrying out the next run. There are several homogeneous and heterogeneous catalytic systems using various metal nitrates and TEMPO to convert HMF into DFF. 154,158,159,161 Homogeneous catalytic systems [M(NO3)x – TEMPO and M(NO3)x – TEMPO – NaNO2] 159 in glacial acetic acid and acetonitrile, respectively, have been developed, which proved to be highly effective for the selective oxidation of HMF to DFF using molecular O2 or atmospheric oxygen as oxidants (Scheme 9). The proposed methods provide a complete conversion of HMF and almost 100% selectivity towards DFF at 50 °C and atmospheric pressure. The investigated catalytic systems were also shown to be promising for the aerobic oxidation of other alcohols.
Scheme 9
Download figure:
Hong et al. 158 prepared an immobilized catalyst [ECS-IL – Al(NO3)3] based on expanded corn starch, silane-modified imidazolium IL, and Al(NO3)3. As an oxidizing agent, O2 was used, and TEMPO as an additive. The catalytic activity of ECS-IL – Al(NO3)3 was confirmed by oxidation of HMF to DFF affording 98% of the target product with 99% conversion of HMF. The obtained catalyst can be recycled, which makes it attractive from economic and environmental points of view.
The proposed catalytic system for converting HMF to DFF, in which ethyl acetate is used as a solvent, 4-AcNH-TEMPO as a catalyst, and Fe(NO3)3 and NaCl as co-catalysts, provided the synthesis of DFF with high selectivity (89% yield). 154 It was noted that due to the poor solubility of 4-AcNH-TEMPO in ethyl acetate, it can be isolated by simple centrifugation and reused as a catalyst (in this case, the DFF yield is 86%).
However, the TEMPO-based catalytic systems free of metals and toxic solvents are more preferable since they most fully comply with the requirements of green chemistry. Kashparova et al. 151 presented an efficient catalytic system for the gram-scale oxidation of HMF to DFF (93% yield) and other alcohols to carbonyl compounds in a two-phase system CH2Cl2 –NaHCO3 (aq.) using iodine as a co-oxidant, catalytic amounts of 4-AcNH-TEMPO, and various pyridine bases (Scheme 10). 2,4,6-Trimethylpyridine (collidine) proved to be the most active co-catalyst, in the presence of which alcohols (37 compounds) were converted into the corresponding aldehydes or ketones in high yields.
Scheme 10
Download figure:
Photocatalytic methods for the oxidation of HMF to DFF have recently become very popular. 129,131,132 Most attractive are so-called metal-free methods using, in particular, graphitic carbon nitride (g-C3N4). 129–131
Thus, photocatalytic aerobic oxidation of HMF in an aqueous medium in the presence of g-C3N4 has been investigated. 129 The achieved 30% selectivity towards DFF exceeded that previously observed with metal-containing catalysts. The samples of g-C3N4 subjected to thermal exfoliation demonstrated enhanced photocatalytic activity and selectivity towards DFF under artificial lighting (42 – 45%). The efficiency of the catalysts increased when natural lighting is used (40% conversion of HMF, 50% selectivity towards DFF). 129 The follow-up studies 130,131 were devoted to further examination and comparison of unmodified (PCN) and modified (PCN-H2O2) polymeric carbon nitride concerning photocatalytic oxidation of HMF to DFF. It was shown that polymeric adduct of carbon nitride and hydrogen peroxide (PCN-H2O2) does not release H2O2 into the reaction aqueous medium upon irradiation, which makes it suitable for photocatalytic application. The study of the mechanism revealed that the exclusion of hydroxyl radicals, localized with H2O2 (Fig. 8), 130 from the reaction process improves the selectivity towards DFF formation under natural lighting from 45 to 88% at a 20% conversion of HMF. Further study confirmed the efficiency of the obtained materials in a pilot plant photoreactor under natural light irradiation. 131
Figure 8. Plausible mechanisms for the formation of (a) hydroxyl radicals involving PCN; (b) adsorption and photooxidation of HMF on PCN; (c) the same on PCN–H2O2. 130 Reprinted with the permission of Elsevier.
Download figure:
Standard imageStudies using traditional materials based on metal oxides and complex metal-containing catalysts for selective oxidation of various organic substrates are in progress.
Selective photocatalytic oxidation of HMF to produce DFF in water using electrochemically generated TiO2 was studied. 132 The observed selectivity towards DFF (⩽33%) for electrochemically synthesized TiO2 nanoparticles is higher than that for the previously reported samples of titanium dioxide obtained by microemulsion and sol – gel methods. Commercial catalyst TiO2 (Evonik P25) is more active in the decomposition of HMF, but its selectivity towards DFF is extremely low (∼1%). This can be due to the higher crystallinity of the commercial sample. Titanium dioxide electrochemically synthesized by electrolysis using pulsed alternating current is predominantly amorphous and has great potential for photocatalysis.
However, it is noteworthy that photocatalytic methods provide significantly lower conversion and(or) selectivity towards DFF as compared to electrochemical and traditional catalytic approaches.
Rodikova and Zhizhina 156 reported a simple procedure to convert HMF into DFF using vanadium-containing heteropolyacids (PMoV HPA), which are highly efficient oxidation catalysts whose activity can be controlled by changing the vanadium(V) content. Optimization of the reaction conditions resulted in a DFF yield of 92% in the presence of Co2H6P3Mo18V7O84 at 110 °C in a two-phase system water – MIBK within 90 min at atmospheric pressure. It was found that the catalytic efficiency of PMoV HPA is determined by their acidity and vanadium(V) content. It was shown that the proposed catalyst can be recycled at least five times without significant loss of catalytic activity.
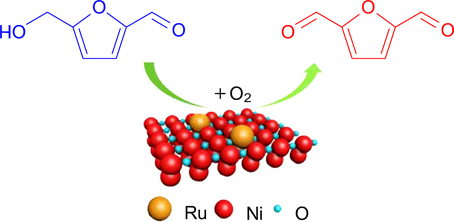
To simplify the preparation of catalysts and improve their catalytic properties, mechanochemical methods have recently been used. 157 Thus, atomically dispersed metal catalysts deposited onto metal oxides, e.g., Ru1/NiO, which were obtained using a ball mill, showed excellent results in selective aerobic oxidation providing HMF conversion of 91.1% and 81.3% selectivity towards DFF at 110 °C in 2 h (Fig. 9). 157 The catalyst can be reused without being aggregated for three catalytic cycles.
Figure 9. Scheme for aerobic oxidation of HMF on a Ru1/NiO catalyst. 157 Reprinted with the permission of the American Chemical Society.
Download figure:
Standard imageTo summarize, the most commonly used methods for obtaining DFF at this time are based on the selective oxidation of purified HMF using both homogeneous and heterogeneous catalytic systems. The straightforward conversion of carbohydrates into DFF, except for fructose, is not without its challenges and does not provide a high yield of the target product. The development of processes for obtaining DFF both from HMF and various carbohydrates using photo- and electrocatalysis would overcome most of the existing limitations. In addition, a more in-depth study of the mechanisms of the DFF formation is important for the development of efficient catalytic systems for its large-scale production.
Despite the fact that DFF is of interest as a monomer and a building block for the preparation of urea – aldehyde resins, polyimines, electroconductive polymers, pharmaceuticals, and other chemical compounds, there are very few modern publications addressing those issues. In this regard, it is pertinent to direct the efforts of researchers towards the development of practical applications of this important platform chemical.
2.6. Synthesis of 2,5-bis(hydroxymethyl)furan and its derivatives
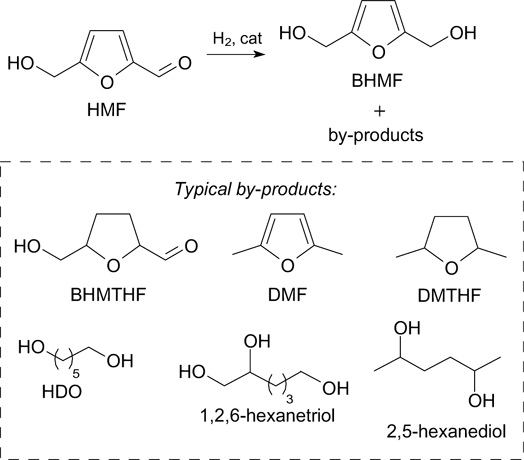
One of the important derivatives of HMF is 2,5-bis(hydroxymethyl)furan (BHMF), which can be obtained by selective reduction of the formyl group in HMF. 16–18,165 In recent years, BHMF has attracted much attention due to its great potential in the synthesis of monomers, polymers, motor fuels, and pharmaceuticals. A review 16 summarizes the state-of-the-art achievements in the production of BHMF from HMF via various chemocatalytic methods such as classical metal-catalyzed hydrogenation, electro- or photocatalytic hydrogenation, disproportionation, and biocatalytic methods. Recent advances in the conversion of BHMF into various valuable derivatives through esterification, polymerization, and rearrangement are also considered.
When carrying out the catalytic hydrogenation of HMF to BHMF using heterogeneous catalysts, by-products of more complete hydrogenation or ring-opening are formed. The main by-product in the hydrogenation of HMF with hydrogen, particularly at elevated temperatures, is bis(hydroxymethyl)tetrahydrofuran (BHMTHF). Also, typical by-products are 2,5-dimethylfuran (DMF), 2,5-dimethyltetrahydrofuran (DMTHF), 1,6-hexanediol (HDO), 1,2,6-hexanetriol, and 2,5-hexanediol (Scheme 11). 16,96
Scheme 11
Download figure:
Research efforts are currently focused on overcoming the difficulties of selective hydrogenation of HMF to BHMF and by-product minimization.
One progressive approach is to replace molecular hydrogen with other hydrogen donors. Catalytic transfer hydrogenation is an effective alternative to traditional catalytic hydrogenation utilizing hydrogen as a reducing agent because it avoids the use of high hydrogen pressure. To carry out hydrogenation via the Meervein – Ponndorf – Verley reaction, hafnium catalysts have been proposed such as a heterogeneous hybrid catalyst (HfCl4 –H3IDC), 166 mesoporous catalyst containing FDCA-Hf salt, 167 allowing the conversion of HMF to BHMF at 100 °C selectively (97 – 98%) in high yield (92 – 98%). The development of more available catalysts is an important challenge. Thus, a novel cost-effective magnetic bimetallic copper – iron nanocatalyst supported on activated carbon (CuO – Fe3O4/AC) has been developed for the selective hydrogenation of HMF in BHMF under Meervein – Ponndorf – Verley conditions using ethanol as a hydrogen donor. 168 The reaction in ethanol with 1 : 1 HMF to catalyst ratio at 150 °C for 5 h gave BHMF with 94.8% selectivity at 97.5% conversion of HMF. The catalyst retained its catalytic activity for five cycles.
Another approach includes optimization of the hydrogenation conditions using traditional heterogeneous catalysts and molecular hydrogen. 18,169 The hydrogenation of aqueous solutions of HMF (2 – 3 mass %) was studied affording two furandiols (BHMF and BHMTHF) with the use of three commercial catalysts (Ru/C, Pd/C, and Pt/C) and a metal loading of 1 mass % in relation to the HMF content. 169 By optimizing the process conditions for Ru/C, BHMF (93%) and BHMTHF (95%) were selectively obtained.
It should be noted that the selective production of BHMTHF by hydrogenation of HMF can also be a big challenge, which was solved by Chen et al. 165 The hydrogenation of HMF to BHMTHF with a selectivity of 96% at 100% conversion was carried out in the presence of a palladium catalyst deposited onto a mesoporous polymer carbon nitride (Pd/mpg-C3N4) in an aqueous medium. Excellent catalytic performance of Pd/mpg-C3N4 is attributed to the competitive hydrogen bonding between HMF and the supported intermediate BHMF (mpg-C3N4), resulting in complete hydrogenation of the BHMF to BHMTHF.
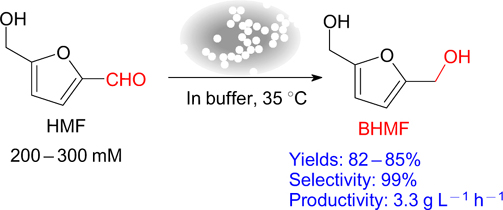
Recently, biocatalytic methods of BHMF production have become very popular, having such advantages as versatility, mild reaction conditions, chemo- and regioselectivity, and a small number of by-products. Biocatalytic transformations involving enzymes, yeast, and plant extracts are known. 167,170–172 Thus, effective approaches to obtain BHMF from HMF using acclimatized Meyerozyma guilliermondii SC1103 cells entrapped in calcium alginate beads have been elaborated. BHMF is formed in 7 – 24 h in a good yield (82 – 85%) and with excellent selectivity (99%) at a substrate loading in the range of 200 – 300 mmol L−1) (Fig. 10). 167 In the presence of recombinant constructed S. cerevisiae yeast strains expressing ADH (MgAAD1669), BHMF was obtained in 94% yield at the substrate concentration of up to 250 mmol L−1 (see Ref. 170).
Figure 10. Scheme for the synthesis of BHMF from HMF using Meyerozyma guilliermondii SC1103 cells. 167 Reprinted with the permission of Elsevier.
Download figure:
Standard imagePetri et al. 171 used fresh or freeze-dried broccoli (Brassica oleracea Italica) to reduce HMF to BHMF. In these experiments, to carry out the reduction of 2 mmol of HMF to BHMF in 91% yield in 48 h, 100 g of the fresh plant material suspended in 250 mL of deionized water was required.
Amarasekara et al. 172 reported the novel effective biocatalytic method for the reduction of HMF in 96% yield using coconut (Cocos nucifera L.) in water at room temperature. The dimer of 5-hydroxymethylfurfural 5,5'-[oxybis(methylene)]bis[2-furaldehyde] can be reduces under similar conditions to afford the corresponding diol 5,5'-[oxybis(methylene)]bis-[2-furanmethanol] in 95% yield. The proposed biocatalytic system can be recycled four times without noticeable loss in catalytic activity.
Despite the attractiveness of biocatalytic processes, they have not yet found wide application because of the lengthy process, the difficulties in storage and handling of enzymes (the need to maintain a certain temperature and pH of the medium).
BHMF is used as a monomer in the preparation of furan polyesters and polyurethanes (see Section 3.3). This can give rise to other key compounds of the furan series. 16 BHMF ethers improve the cetane number for diesel, 173 BHMF esters with unsaturated acids are used as monomers, 174 BHMF esters substitute a potential substitute for phthalate plasticizers; 175 ammonolysis of BHMF gives the corresponding amino derivatives. 176
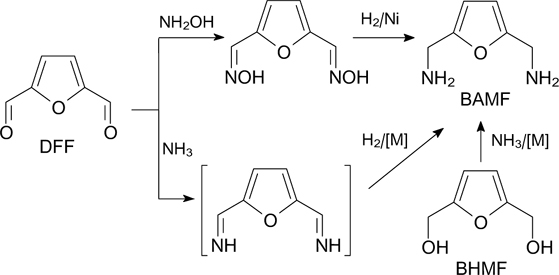
Starting from BHMF, one of the promising furan monomers can be synthesized, namely, 2,5-bis(aminomethyl)furan (BAMF), which is used in the synthesis of furan polyamides. 176 The main synthetic approaches to BAMF include the reductive amination of DFF or ammonolysis of BHMF (Scheme 12). 1,177 As a catalysts for reductive amination of DFF, e.g., Raney nickel can be used (42.6% yield of BAMF). 176 Ruthenium complexes were used for ammonolysis of BHMF (46.8% yield of BAMF). 178
Scheme 12
Download figure:
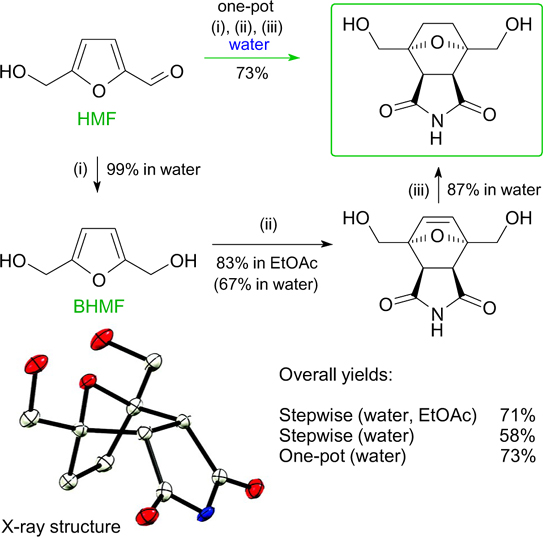
The development of new approaches to the synthesis of complex derivatives based on HMF and BHMF is an important objective. Noteworthy in this regard is the Diels – Alder reaction. Reactivity of HMF and BHMF in the Diels – Alder synthesis was evaluated using computational modelling. 179 The results showed that unsubstituted BHMF is more reactive towards maleimide than HMF. Preliminary theoretical calculations helped to evaluate the possibility of synthesis of three-dimensional polycyclic structures based on HMF and BHMF (Fig. 11). This is the first example of the Diels – Alder reaction between unsubstituted BHMF and maleimide being carried out in water with high stereoselectivity. The developed approach was extended to functionalized amines, ethers and esters containing a furan nucleus, which opens up new possibilities for the synthesis of demanded furan derivatives. 179
Figure 11. Scheme for the synthesis of bis(hydroxymethyl)norcantharimide in water. (i) NaBH4, water; (ii) maleimide, water; (iii) H2, 0.1 MPa, 10% Pd/C or Ni/Ra, water. 179 Reprinted with the permission of the Royal Society of Chemistry.
Download figure:
Standard imageTo conclude, the researchers consider catalytic transfer hydrogenation as an effective alternative to the traditional catalytic hydrogenation of HMF to BHMF using hydrogen, since it is safer and does not require drastic reaction conditions. Various heterogeneous catalytic systems, both classical bimetallic (Cu-Ni, Ni-Fe, Co-Ru) and containing organic ligands combining sites of Lewis acidity and basicity, demonstrate high efficiency in catalytic transfer hydrogenation reactions. Despite the progress achieved in this area, classical methods of catalytic hydrogenation using molecular hydrogen are still more suitable for industrial application. In this case, research efforts are directed towards optimizing the hydrogenation conditions using traditional heterogeneous catalysts.
2.7. Synthesis of levulinic acid and its derivatives
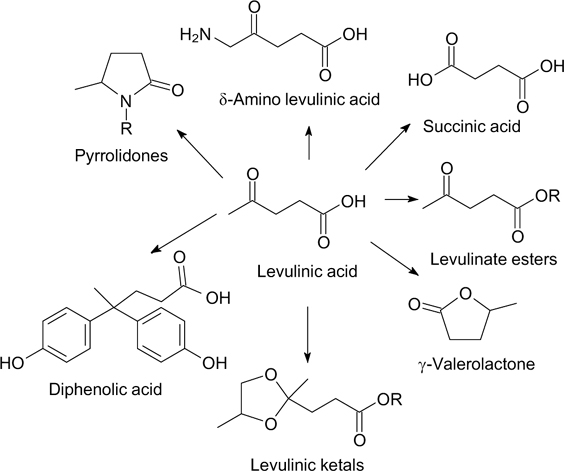
Levulinic acid (LA) is another versatile platform chemical representing the basis for a plethora of chemical compounds. LA can be obtained from renewable resources such as sugars, lignocellulosic biomass, and waste by catalytic conversion of carbohydrates to HMF. The latter compound transforms into LA and formic acid under the action of acids (sulfuric, hydrochloric, trifluoroacetic, etc.) and water. 2,180 In turn, the potential for obtaining valuable chemical compounds based on LA is high due to the presence of both carbonyl and carboxyl groups in its molecule. Recently, laboratory-scale synthesis of LA and its derivatives has been investigated using homogeneous or heterogeneous catalysts. 19,20,180,181 The availability of LA opens up prospects for obtaining valuable derivatives, in particular esters, methyltetrahydrofuran, γ-valerolactone (GVL), diphenolic acid (DPA), succinic acid, etc., (Fig. 12). 19
Figure 12. Most important derivatives of levulinic acid. Adapted from the data of Ref. 19.
Download figure:
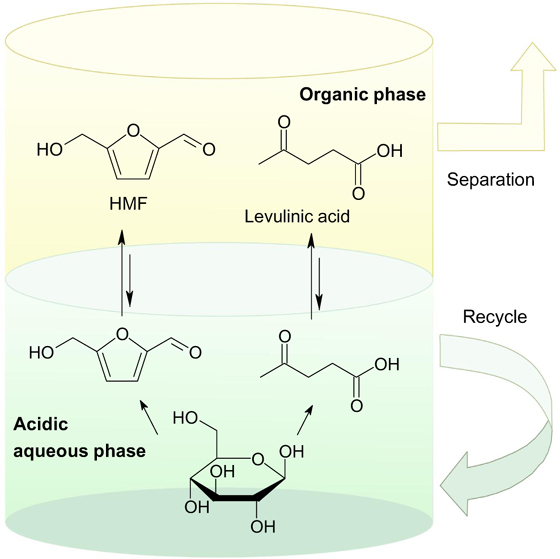
Standard imageA recent review 20 considers the latest developments in the production of HMF and LA from lignocellulosic biomass using both homogeneous and heterogeneous catalysts in organic solvents and two-phase systems. Although homogeneous catalysis provides the highest yield of LA, recycling of homogeneous catalysts is a major challenge. In this respect, it is advantageous to use two-phase reaction systems (Fig. 13).
Figure 13. Two-phase systems for dehydration of carbohydrates to obtain valuable chemical compounds such as HMF and LA. 20 Reprinted with the permission of Elsevier.
Download figure:
Standard imageStudies of the reaction mechanism for the LA formation from various raw materials are of great importance for a deeper insight into the process at the molecular level, the development of strategies for increasing the yield of LA, and suppressing the formation of by-products.
The mechanism for the HMF formation via dehydration of D-fructose and subsequent generation of LA and formic acid from HMF through rehydration is studied by in situ13C and 1H NMR using both usual and 13C-labelled fructose.
182
Water or DMSO was used as a solvent, and Amberlyst 70,  acid or sulfuric acid were employed as catalysts. HMF is formed through dehydration of fructose in DMSO with any of the three catalysts or without any catalyst. Levulinic and formic acids were formed only in the presence of water, which is consistent with the general mechanism for HMF rehydration, thereby the C(1) and C(6) carbon atoms of HMF are being transferred into formic acid, and C(5) carbon atom moves to levulinic acid, respectively.
182
acid or sulfuric acid were employed as catalysts. HMF is formed through dehydration of fructose in DMSO with any of the three catalysts or without any catalyst. Levulinic and formic acids were formed only in the presence of water, which is consistent with the general mechanism for HMF rehydration, thereby the C(1) and C(6) carbon atoms of HMF are being transferred into formic acid, and C(5) carbon atom moves to levulinic acid, respectively.
182
Studies have confirmed 183 that strong acids can catalyze the conversion of hexoses (glucose, fructose) or cellulose, as well as lignocellulosic biomass into LA and formic acid in an aqueous solution over a temperature range of 140 – 225 °C. The most efficient acidic catalysts are sulfuric, hydrochloric, and trifluoroacetic acids at a concentration from 0.1 to 2 mol L−1. Glucose pre-isomerizes to fructose, dehydrates to give HMF, and then transforms rapidly into LA and formic acid. Glucose is a good and available feedstock for the production of HMF and LA due to its low cost. Nevertheless, as a renewable carbon source, lignocellulosic biomass is a more preferable feedstock for the synthesis of HMF and LA. 20 However, the methods of obtaining LA from mono- and polysaccharides are still highlighted in the literature.
Starting from LA and LA lactone, new-type biodegradable polymeric materials were prepared. 184,185 Lactone of LA, so-called α-angelica lactone [5-methyl-2(3H)-furanone], bears two functionalities that can enter polymerization reactions. There are two possible ways of polymerizing α-angelica lactone: the double bond opening to afford polyfuranone and lactone ring opening to give a polyester (Scheme 13). 184 The polymer yield in anionic polymerization is 41 – 45%. 185
Scheme 13
Download figure:
A convenient and effective method for the production of LA from cellulose in IL functionalized with SO3H groups has been proposed. 186 The effect of IL structures, reaction conditions, and the combinations of metal chlorides with ILs on the LA yield were studied; the highest yield (39.4%) was obtained in 2 h in the presence of 1-(4-sulfo)butyl-3-methylimidazolium hydrogen sulfate ([BSmim]HSO4) with H2O added. Ionic liquids functionalized with SO3H groups serve both as a solvent and as an acidic catalyst in converting cellulose into LA, and retain their catalytic activity for four cycles.
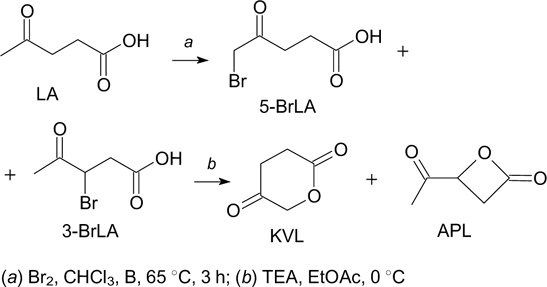
A synthetic approach to 4-ketovalerolactone (KVL) starting from LA has been proposed (Scheme 14). 187
Scheme 14
Download figure:
Ring-opening transesterification polymerization (ROTEP) of 4-ketovalerolactone affords poly(4-ketovalerolactone). Polymerization of KVL proceeds with a high monomer conversion (up to 96% in the melt) to produce semicrystalline poly(4-ketovalerolactone). This polyester has a glass transition temperature of 7 °C and two melting points (132 and 148 °C) and can be chemically processed via hydrolytic degradation. 187
Non-catalyzed and Novozym 435 enzyme-catalyzed esterification reactions of levulinic acid with methanol, ethanol, and 1-butanol in a wide temperature range (323 – 473 K) were studied. 188 Thermodynamic analysis of the reaction was carried out using quantum chemical methods and PC-SAFT simulation. It was demonstrated that experimental data are in good agreement with the results of G4-calculations. An appropriate mix of quantum chemical methods and PC-SAFT simulation with empirical methods helps to reduce experimental efforts to evaluate the feasibility of chemical processing of renewable raw materials.
To summarize, levulinic acid is obtained both from sugars (fructose, glucose, cellulose) and directly from lignocellulosic biomass with the use of various ILs, which reduces the reaction temperature as compared to the synthesis in an organic solvent. However, due to the high cost, the use of ILs as solvents in large-scale production is economically unsustainable. Although homogeneous catalysis provides the highest LA yield, the recycling of homogeneous catalysts is a serious problem. This issue can be addressed by the use of two-phase reaction systems. Further research of the reaction mechanism for LA formation from various raw materials is of great importance for a better understanding of the process at the molecular level, which will contribute to the development of a strategy for increasing LA yield and minimizing by-products.
3. Polymer materials based on furanic monomers
Currently, polymers and composite materials based on furan monomers play a key role in industry and everyday life. Society has become more dependent on plastic materials since the beginning of their wide industrial production in the middle of the 20th century. Versatility, stabilily, low weight, and low production costs have contributed to the increase of global demand for polymers. 189,190 However, plastic materials pose serious environmental challenges. For example, almost all of the plastic packaging manufactured (including plastic bottles) — more than 80 million tons annually — is produced from fossil resources and disposed of after a relatively short period of use. 21,191 While more and more plastic is recycled or burned to replenish energy, most ends up in landfills in or outside cities and also in oceans 21,192,193 (Fig. 14). 194
Figure 14. World production, utilization, and fate of polymers produced from 1950 to 2017 (million tons). 194 Reprinted with the permission of Elsevier.
Download figure:
Standard imageThe production of polymers from biomass, and especially biodegradable polymers, can solve many of the environmental problems associated with petrochemical production and make plastics production sustainable and environmentally acceptable. 21–23
3.1. Polymers based on 2,5-furandicarboxylic acid and its derivatives
Among available furan compounds, FDCA, 2,10–14,95,124,195 which can become a viable alternative to various petroleum phthalic acids in the production of polyesters, polyamides, and polyesteramides based on renewable plant raw materials, is of special interest.
3.1.1. Synthesis of polyesters
The synthesis of various polyesters based on FDCA and its derivatives has been the focus of numerous works over the past 5 – 10 years. 6,24,99–101,196–206
Zhang et al. 207 provided the most comprehensive classification of the advances and challenges in the development and study of the properties of such polyesters (including PEF, PPF, PBF, PHF). Prospects for the production and use of biopolymers such as polylactide (PLA) and PEF, which are becoming increasingly popular due to a combination of valuable properties, are considered in a mini-review. 205 A comparison of these biopolymers with the most common analogues obtained from fossil raw materials, namely, PET and polybutylene terephthalate (PBT), is carried out.
PEF is considered a non-toxic, biocompatible material that represents an alternative to one of the most common polymers, petroleum-based PET. PEF is prepared by the reaction of FDCA or its derivatives with ethylene glycol. 203,205,208 Various companies have been working on the development of industrial processes for PEF production for more than 10 years. 1,53,96
Polyethylene furanoate attracts the attention of researchers and manufacturers due to its unique properties, such as lower gas permeability and excellent thermophysical characteristics compared to PET traditionally used for the production of bottles and packaging. Thus, the barrier properties of PEF against O2 and CO2 exceed those for PET by 6 – 11 and 11 – 19 times, respectively (depending on the method of producing of a polyester). 100 The glass transition temperature of PEF is 80 – 88 °C (4–12 °C higher than that of PET), therefore, PEF is more thermally stable. PEF has a melting point of 210 – 230 °C (20–40 °C lower than that of PET), making it easier to recycle. Its modulus of elasticity is almost 1.5 times that of PET. This polymer can be processed into fibres, yarns, films, and various containers. 197,199
In 2009, it was shown that high molecular weight PEF, the properties of which are comparable with those of PET, can be successfully synthesized. 208 This has led to a renewed interest in this material, especially considering that the use of biomass for the synthesis of not only FDCA but also ethylene glycol makes it possible to produce PEF entirely on a biorenewable basis.
Further efforts were aimed at expanding the scope of research on the synthesis of furan polyesters in terms of (i) searching for alternative methods of synthesis and catalysts to replace the rather toxic Sb2O3, 99,195,200,203 (ii) characteristics of the mechanical and thermophysical properties of PEF compared to PET, 197,199 (iii) the possibility of using other polyols, such as propylene glycol, butylene glycol, glycerol, hexanediol, etc., 196,198 as well as to obtain various copolymers and mixtures of PEF with other polymers. 99,200,209
Condensation of FDCA derived fron renewable raw materials with glycerol in the presence of 2 mol.% of catalyst at 210°C gave branched polyesters in ∼ 70% yield. 198 The product, which is a solid resin, is insoluble in common organic solvents and slightly soluble in trifluoroacetic acid. The synthesis of novel furan polyesters based on a dimeric furan acid such as 5,5'-[oxybis(methylene)]bis[2-furancarboxylic acid] (OBFA) was reported. 210 Along with FDCA, the new monomer can be considered as an alternative to terephthalic acid, as demonstrated by the example of the preparation of polyesters with ethylene glycol and 1,4-butanediol in a yield of 87 – 92%.
An efficient laboratory method for PEF synthesis has been developed, which can be scaled up to a small-scale production. Polyester in 95 – 98% yield was synthesized via two-step melt polycondensation in a glass batch reactor using 0.04 mol.% of Zn(OAc)2 or Ti(OBun)4 at a diester to diol ratio of 1 : 2.2. 203
The synthesis of polyesters based on furan acids has recently been aimed at obtaining co-polyesters containing, along with FDCA, other acids, for example, ε-hydroxycaproic acid, and other diols such as pentane- or hexanediol. This can impart specified physico-mechanical and functional characteristics (glass transition, melting, and decomposition temperatures; Young's modulus, crystallinity degree, heat resistance, etc.) to the polymers. 99,206,211,212 Terzopoulou et al. 213 presented the state-of-the-art trends in the synthesis of the FDCA-based co-polyesters and the study on the viability of this approach in converting them into a more versatile class of materials that are potentially suitable for a number of high-tech and traditional applications.
Thus, two series of co-polyesters, namely, poly(pentylene 2,5-furandicarboxylate-co-caprolactone) (PPeCF) and poly(hexamethylene 2,5-furandicarboxylate-co-caprolactone) (PHeCF), have been successfully obtained via the reaction of ε-caprolactone (CL) with poly(pentylene 2,5-furandicarboxylate) (PPeF) and poly(hexamethylene 2,5-furandicarboxylate) (PHF) at various molar ratios of components. 99 These materials, with the CL content ranging from 10 to 50 mol.%, were first obtained under the catalysis of stannous octoate. The tests demonstrated the good thermal stability of novel co-polymers exceeding 310 and 360 °C for PHeCF and PPeCF, respectively.
Based on FDCA, not only saturated polyesters like PEF, PBF, and PHF can be synthesized, but also unsaturated polyesters capable of cross-linking when using unsaturated diacids (maleic, itaconic) as co-monomers. 206,211,212
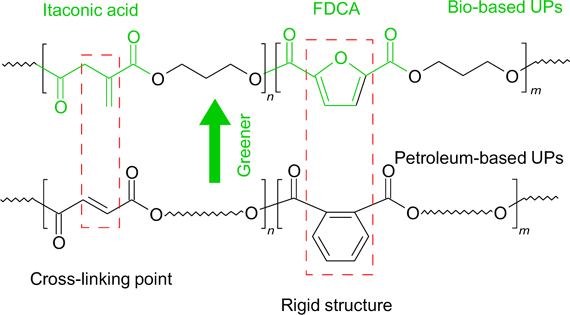
A series of bio-based unsaturated polyesters (UPs) was produced by the direct polycondensation between FDCA, itaconic, succinic acids, and 1,3-propanediol (Fig. 15). 211 The test results showed that the thermal and strength properties of these cured polyesters significantly improved after the introduction of FDCA, the heat resistance reached 330 °C, and the flexural strength and modulus of elasticity were 122.8 and 3521 MPa, respectively.
Figure 15. Comparison of structures of unsaturated polyesters based on FDCA and terephthalic acid. 211 Reprinted with the permission of the American Chemical Society.
Download figure:
Standard imageThe synthesis of an unsaturated polyester resin with good thermal properties have been developed and its characteristics were studied. 212 The process was carried out with various ratios of FDCA, itaconic acid, and diols. An increase in the FDCA content has a positive effect on the thermal properties of polyesters, the glass transition temperature (Tg) of which rises to 30 – 32 °C. The possibility of replacing styrene, which is usually used as a diluent and cross-linking agent, with a safer alternative for cross-linking polyester resin, namely, bio-based dimethyl itaconate and butanediol dimethacrylate, has been studied. After cross-linking with dimethyl itaconate, the average Tg was 75 °C [which is higher than with butanediol dimethacrylate (42 °C)]. The development of such resins opens the way to the application of bio-renewable monomers in the industrial production of thermosetting polyesters.
Unsaturated polyesters obtained with the use of FDCA and maleic anhydride, and phthalic anhydride, adipic or sebacic acids, are reported. 206 The effect of the acidic component ratio in the composition of glycol – (dicarboxylic acid + maleic anhydride) on the properties of polyesters and composites based on them was studied. Composites based on FDCA and maleic anhydride as the acidic component and diethylene glycol as an alcohol component have excellent strength properties, which significantly exceed the strength of composites based on other dicarboxylic acids. A preliminary study was carried out to attempt the preparation of bio-maleic anhydride from HMF by oxidizing the latter in an aqueous solution under mild conditions in the absence of toxic catalysts and solvents. It was shown for the first time that maleic acid can be prepared in quantitative yield by oxidation of HMF in an aqueous solution with hydrogen peroxide in the presence of sodium bicarbonate at room temperature and atmospheric pressure.
A promising area in polymer chemistry is enzymatic polymerization giving an excellent opportunity to effectively convert renewable resources into polymer materials without the use of toxic metal-containing catalysts (Fig. 16). 102,214–216
Figure 16. Benefits of enzymatic polymerization in the production of bio-renewable polymers. 214 Reprinted with the permission of MDPI AG.
Download figure:
Standard imageThe synthesis of various furan polyesters via enzymatic polymerization is considered. 200,215,216 Polycondensation of BHMF with several dicarboxylates has been investigated and the structure of the dicarboxylic acid was found to affect the thermal stability and crystallinity of the products, with both parameters increasing with an increase in the number of methylene units in the polyester chain. 215 An enzymatic reaction between FDME and aliphatic diols was carried out to elucidate the effect of diol structures on polyester properties. 216 A series of furan-based co-polyesters with a molecular weight up to 35 kg mol−1 have been successfully synthesized using Novozym 435 as a biocatalyst, FDME, BHMF, aliphatic linear diols (C4 –C14) and ethyl esters of various diacids (C4 –C14) as monomers. 200 All resulting co-polyesters are semicrystalline materials except for poly(2,5-furandimethylene furanoate-co-2,5-furandimethylene succinate) and poly(2,5-furandimethylene furanoate-co-2,5-furandimethylene adipate). This suggests that the presence of 2,5-furandimethylene furanoate unit in the backbone hampers crystallization of co-polyesters.
To improve the thermophysical properties of bio-based polymers, not only various copolymers but also polymer blends were prepared and tested. 24,202 Thermoplastic furan-based polyesters (PEF, PPF, PBF) and poly (1,4-cyclohexanedimethylene 2,5-furandicarboxylate) (PCHDMF) were used to prepare blends with polymers of industrial importance, including PET, poly(ethylene-2,6-naphthalate) (PEN), PLA, and polycarbonate (PC). 24 Studies have shown that PEF blends generally had two glass transition temperatures for the resulting samples. Only PPF – PEF blends had a single glass transition temperature and a homogeneous melt phase according to polarized light microscopy data. PPF forms poorly miscible blends with other polymers except for PEF and PBF. Polybutylene furanoate forms homogeneous blends with PCHDMF and PPF, whereas other mixtures are characterized by two glass transition temperatures and phase separation in polarized light. PCHDMF – PEF and PEN – PEF mixtures also have two different Tg, but they shift to intermediate temperature value (in the range of Tg values for pure polymers) depending on the composition, which indicates some partial miscibility of polymer pairs.
Polymer blends of PET, modified with recycled glycol (PET-G), and PEF, synthesized from FDCA dimethyl ester and ethylene glycol (BioUltra), were prepared and tested. 202 Using calculations by Hoy's method, compatibility of the blend components was studied. Tensile tests demonstrated that an increase in Young's modulus was observed with increasing PEF content. Also, interfacial interactions between polymers, especially in the PET-G – PEF (80 : 20) blend, resulted in the formation of a network structure between the PET-G and PEF chains, providing a synergistic effect with improved thermal stability and decrease in water absorption.
The researches focus on practical application of furan polyesters, above all, PEF. 101,204 Thus, biomass-derived PEF was used for 3D printing. 101 A complete cycle from dehydration of cellulose to finished products was performed. Parts made from PEF showed higher chemical resistance than those printed using commonly available materials [acrylonitrile butadiene styrene plastic, PLA, glycol-modified PET (PET-G)]. PEF demonstrated good suitability for 3D printing: optimal adhesion, thermoplasticity, no delamination and low thermal shrinkage, and also reusability. The proposed approach to using PEF for additive manufacturing opens a new trend in the field of sustainable development.
A conceptual approach to the design and use of new polymer materials such as PEF and PHF obtained from renewable raw materials has been proposed. 204 The transformation of a complex irregular structure of a natural biopolymer into a hierarchically well-defined polymer chain of furan polyesters led to the discovery of unique adhesive properties of the obtained materials, enhanced by a new effect of multiple binding with the participation of oxygen of the furan ring. Thermoplastic reusable adhesives based on PEF and PHF were prepared and studied, which have shown exceptional adhesion to aluminium in shear tests (1.47 ± 0.1 and 1.18 ± 0.1 kN cm−2, respectively), and to glass surfaces (0.93 ± 0.11 kN cm−2 for PEF and 0.84 ± 0.06 kN cm−2 for PHF) and to other materials. The developed reusable hot-melt adhesive is universal and suitable for various materials such as copper, brass, Be-copper, Mn-bronze, aluminium, titanium, and glass.
Due to its similarity to PET, PEF is currently the most important polyester derived from FDCA. It is expected that PEF will be commercially available by 2023. 217 However, despite the many excellent qualities of PEF, it also has disadvantages such as toughness, brittleness, and difficulty in processing. To overcome these constraints, methods of production of various FDCA-based copolyesters and blends of furan polyesters or polyamides with various commercial polymers (PET, PEN, PLA, PC) are being developed and their properties are being studied to impart new physical, mechanical, and functional properties to polymers. It should be noted that in search for the most environmentally friendly methods for the synthesis of furan polyesters, enzymatic methods come to the fore, as they provide polymer materials under mild conditions in the absence of toxic metal-containing catalysts.
3.1.2. Synthesis of polyamides and polyimides
2,5-Furandicarboxylic acid is a basis for preparing not only polyesters, but also aromatic and fatty-aromatic polyamides. The method of direct melt polycondensation of FDCA with aliphatic and aromatic diamines is the most effective in terms of tacticity, high molecular weight, and high thermal stability of the resultant polymers. 103,218 The method of interfacial polycondensation in an aqueous-organic medium using FDCA dichloride (Scheme 15) is also used, as avoiding the use of toxic solvents and high temperatures. 219
Scheme 15
Download figure:
Polyamides based on furan monomers have a great potential for producing materials with high thermal, mechanical, and performance characteristics. The aromatic nature of the furan ring makes it an attractive alternative to aliphatic moieties since the aromatic furan ring is more rigid than cyclohexane and more polar as compared to benzene. As a consequence, FDCA-derived polymers outperform aliphatic and cycloaliphatic polyamides, and at the same time are equal to aromatic polyamides. 103 Novel polymers could be an alternative to such materials as poly(p-phenylene terephthalamide) and poly(m-phenylene isophthalamide) (trade names Kevlar and Nomex, respectively). However, much less attention has been paid to the synthesis of polyamides based on FDCA and its derivatives than to the synthesis of the corresponding polyesters. Therefore, the development of sustainable methods for the synthesis of polyamides based on renewable plant biomass is an urgent challenge. 102,103,214,220–223
Recently, research on the synthesis of polyamides has focused on the use of both monofuran monomers (FDCA, FDME, FDCDCl, BHMF, BAMF, 2-furamide) and dimeric furan derivatives (Fig. 17), as well as on the design of co-polyamides. 103,218,224,225
Figure 17. Structures of monomers containing two furan moieties. 103
Download figure:
Standard imageThe synthesis of a series of aromatic furanic polyamides based on FDCA and various aromatic diamines (p-phenylene-, o-phenylenediamine, 4,4'-diaminodiphenyl oxide, 4,4'-diaminodiphenylmethane) via direct polycondensation was described, and the properties of the resultant compounds were studied. 218 The obtained polyamides displayed good solubility in organic solvents and good thermal and mechanical characteristics.
The solvent nature was shown to affect the course of the polycondensation and the properties of polyamides obtained from FDCA and p-phenylenediamine using triphenyl phosphite as a condensing agent. 225 The higher thermal stability and degree of crystallinity of the polyamide derived from N,N–dimethylacetamide, as compared to NMP, is due to its higher solvating ability.
Two approaches to the preparation of furanic polyamides have been investigated: (i) synthesis of hexamethylenediamine furanoate salt and its subsequent polycondensation, and (ii) synthesis of FDCA dichloride and its subsequent polycondensation with hexamethylenediamine or p-phenylenediamine in a water-organic medium. 222 The effect of the nature of organic solvent (tetrachloromethane, dichloromethane, NMP) and diamine (hexamethylenediamine and p-phenylenediamine) on the yield and molecular weight of polyamide was studied. The prepared polymer was used for producing enamels with various fillers, such as colloidal graphite or activated carbon, obtained from biomass processing waste in HMF (see Section 4). The furanic polyamide coatings on steel have a lower friction coefficient and lower wear as compared to coatings from commercial polyamide (PA-6) and can be considered as novel promising materials for antifriction coatings (Scheme 16).
Scheme 16
Download figure:
When using FDCA dichloride and hexamethylene-, 1,4-phenylene-, 1,2-phenylene-4-methyldiamine, and 4,4-diaminodiphenylmethane, the corresponding polyamides with good heat resistance were obtained. 223 Studies have shown that the obtained polyamides have the decomposition temperature in the range of 400 – 420 °C, except for the polyamide derived from 4,4-diamino-diphenylmethane, the decomposition temperature of which is below 400 °C.
As in the case of obtaining furanic polyesters, methods of biocatalytic polycondensation are of particular interest. Thus, poly(octamethylene furanamide) (PA8F), an analogue of poly(octamethylene terephthalamide), was successfully obtained via a one-step polycondensation catalyzed by Novozym 435 in toluene, and a two-step reaction in diphenyl ether, respectively. 224 Enzymatic polymerization gives PA8F with a high average molecular weight (up to 54 000 g mol−1). Studies of the one-step enzymatic polymerization in a solvent at 90 °C revealed that the molecular weight of PA8F grows up significantly with increasing concentration of Novozym 435. The two-step enzymatic polymerization at different temperatures in diphenyl ether yields PA8F with higher molecular weights as compared to the one-step procedure.
An enzymatic method to obtain polyamide (PA5F) from FDME and 1,5-pentanediamine has been elaborated. 226 The reversibility of the PA5F cross-linking process using the Diels – Alder reaction was shown. The material was cross-linked at a low temperature, and then returned to its original state when heated. As an alternative to the thermal impact, ultrasonication was used, which demonstrated greater efficiency in the onset of the regeneration into the starting polymers.
Along with FDCA-based polyamides, other important materials such as polyimides (PI) and polyamidimides (PAI) are synthesized. Aromatic PIs and PAIs are considered as one of the most advanced classes of polymer materials with good performance, high melting point, and chemical resistance. 227–229
Bio-polyimides derived from novel furan-based diamines N,N'-bis(3-aminophenyl)furan-2,5-dicarboxamide (m-FDDA) and N,N'-bis(4-aminophenyl)furan-2,5-dicarboxamide (p-FDDA) were obtained through a two-step polycondensation with commercially available aromatic dianhydrides (BPDA, 6FDA, BPADA) (Fig. 18). The resultant PIs are characterized by high glass transition temperatures (Tg = 266 – 364 °C), high thermal stability (T5% = 420 °C), and good mechanical properties (tensile strength of 79 – 138 MPa, modulus of elasticity of 2.5 – 5.4 GPa). Common features of PIs derived from FDCA are comparable with those for PIs based on isophthalic acid thus demonstrating the potential of the development of innovative bio-based polyimides.
Figure 18. Structural formulas of furan diamines (m-FDDA, p-FDDA) and commercial dianhydrides (BPDA, 6FDA, BPADA). Adapted from the data of Ref. 229.
Download figure:
Standard imageA series of cross-linked polyimides were synthesized from FDCA, 2,2-bis[4-(3,4-dicarboxyphenoxy)phenyl]propane dianhydride (BPADA) and m-FDDA or p-FDDA diamines via a twostep polycondensation in solution followed by thermal cross-linking with bismaleimide. 230 The cross-linked film showed improved solvent resistance, thermal (Tg = 234 – 306 °C) and mechanical properties (tensile strength of 82 – 98 MPa, modulus of elasticity of 2.3 – 3 GPa).
To improve thermal and mechanical characteristics of furan polymers, semicrystalline poly(esteramide) were synthesized from FDCA, 1,10-decanediol, and amidodiol, which is a symmetric diol having two internal amide bonds. 231 The degree of crystallinity, thermal and mechanical parameters of the obtained polymers can be adjusted by changing the ratio of 1,10-decanediol and amidodiol in the initial mixture. In this case, the glass transition temperature (Tg) and melting point (Tm) of poly(decylene furanoate) rise from –4 to 27 °C and from 102 to 175 °C, respectively. Modulus of elasticity and hardness also increase significantly with an increase in the number of amidodiol moieties in the main chain.
Polyamides containing furan moieties are still considered as a promising biorenewable alternative to petroleum-based polyamides, since FDCA-derived polyamides are superior in physical and mechanical properties to aliphatic and cycloaliphatic polyamides, and are not inferior to aromatic polyamides. However, to organize their large-scale production, several important issues need to be addressed: to make high purity FDCA available, to develop new strategies for the synthesis of furan polyamides with a high molecular weight and narrow molecular-weight distribution, to develop new types of furan derivatives as monomers, using them for the synthesis of polyamides and studying the structure – properties relationships for new bio-based polymer materials.
3.1.3. Synthesis of other polymers
Starting from FDCA and 5,5'-[hydroxybis(methylene)]-bis-[2-furancarboxylic acid] (OBFA), monomers can be obtained containing unsaturated groups capable of entering the polymerization reaction. 26,232–234 Unsaturated furan-based derivatives were synthesized, namely, diallyl (DAF) 26,232 and dicinnamyl (DCF) 233 esters of FDCA, diallyl ester of OBFA (DADF), 26,232 which can be used as monomers and cross-linking agents. DAF, DADF, and DCF virtually do not enter the homopolymerization reaction (which is due to the stability of the radicals derived therefrom), but perfectly copolymerize with various vinyl monomers. Butyl methacrylate (BMA), styrene, acrylic (AAc), and methacrylic (MAAc) acids were utilized as comonomers. Depending on the composition and degree of cross-linking, the resultant copolymers can be applied as adhesives (copolymers with BMA) and construction materials (copolymers with styrene). By varying the amount of cross-linking agent in copolymers, one can adjust thermal stability, adhesion, strength, optical properties, exchange and swelling capacities of materials. Copolymers of DAF and DADF with AAc or MAAc exhibited excellent water absorption and ion-exchange properties, good adsorption of heavy metals (Fig. 19). 26 Their maximum adsorption capacity for NiII, CoII, and CuII was 365, 360, and 290 mg. g−1, respectively, which is 1.5 – 2.6 times higher than that of commercial ion-exchange materials such as Amberlite IRC-748 and Purolite C-106.
Figure 19. Kinetics of adsorption of AAc/DADF copolymers for different concentrations of NiII (a), CoII (d) and CuII (g). Pictures of resins before and after adsorption (b, e, h). Isotherms of adsorption of NiII (c), CoII (f), CuII (i) by copolymers AAc/DAF, MAAc/DAF. 26 Reprinted with the permission of Wiley.
Download figure:
Standard imageOxidation of DAF gave FDCA diglycidyl ester, which was used to produce epoxy resins. 234 The use of this monomer provided a higher curing activity of epoxy oligomers and an increased glass transition temperature of the obtained polymers. Mechanical properties of cured bio-epoxides are just as good as those for similar polymers based on diglycidyl terephthalate, which indicates that FDCA is promising for the synthesis of bio-epoxides.
It should be pointed out that expanding the range of furan monomers of the series through compounds containing unsaturated groups, for example, allyl, or conformationally flexible rings, for example, epoxy ones, opens access to novel furan polymers and copolymers with a set of useful properties. Thus, copolymers of furanic diallyl dicarboxylates (DAF, DCF, DADF) with various vinyl monomers (styrene, BMA, MMA) have good physical and mechanical characteristics, while those containing the units of unsaturated acids such as MMAc or AAc, depending on the content of the cross-linking agent, have a high water-absorbing and ion-exchange capacities, and also display antimicrobial activity. These materials are of practical interest for various industries, including water treatment, agriculture, and medicine. Further research in the area of synthesis and study of the properties of unsaturated furan-based derivatives will contribute to the development of novel polymers with various, possibly unique, properties.
3.2. Polymers based on 2,5-diformylfuran and its derivatives
Another promising building block for polymer production is 2,5-diformylfuran (DFF) containing two aldehyde groups. It can be used to synthesize urea – aldehyde resins, 145 polyimines, 146 electroconductive polymers, 147 and porous polymer structures. 148
By melting a solid mixture containing DFF and urea, a crystalline urea – aldehyde resin was obtained in a 90% yield. 145 The structural unit of the polymer is one DFF molecule condensed with two urea molecules (Scheme 17). Using a similar procedure, a homologous resin was obtained based on 5,5'-[oxybis(methylene)]bis[2-furaldehyde] and urea in 84% yield.
Scheme 17
Download figure:
The possibility to prepare porous polymer structures via condensation of DFF with various diamines was investigated. 148 Porous polyimines are insoluble in water and organic solvents and retain their thermal stability up to 300 °C in an inert atmosphere. Materials obtained from aromatic amines, in which NH2 groups are located in meta-positions, have higher specific surface areas and micropore volumes, in contrast to polymers based on aromatic 1,4-diamines, and demonstrate a high adsorption capacity for CO2 (77 mg g−1).
The synthesis of degradable DFF-based poly(silyl ethers) with an average molecular weight up to 25 000 g mol−1 and thermal stability to 473 °C has been reported. 235 A manganese complex [MnN(salen-3,5-But 2)] was used as a catalyst. Dhers et al. 236 synthesized a polyimine by the reaction of DFF with a mixture of bio-based di- and triimines without using a catalyst (Fig. 20). The effect of the solvent on the molecular weight and properties of polymers was investigated. The resultant materials have a high relaxation capacity at room temperature and good acid and alkali resistance, thus allowing their application as construction materials for operation in harsh environments.
Figure 20. Synthesis of polyimine from DFF and a mixture of di- and triamines. 236 Reprinted with the permission of the Royal Society of Chemistry.
Download figure:
Standard imageIt should be noted that the DFF potential as the monomer is not fully recovered yet; there is a limited number of new works concerning the development of polymers based on it, as well as DFF-derived electroconductive polymers.
3.3. Polymers based on 2,5-bis(hydroxymethyl)furan
2,5-Bis(hydroxymethyl)furan (BHMF) bears two hydroxyl groups, therefore it can be used for the synthesis of polyesters and polyurethanes 237–240 with valuable properties, including a 'self-healing' ability. 237,239
Zeng et al. 237 synthesized a polyester based on BHMF and succinic acid and carried out the cross-linking of the former using 1,8-bis-(maleinimido)triethylene glycol via the Diels – Alder reaction. This polymer has self-healing properties upon treating the damaged surface with a cross-linking agent solution or solvent (TGF), and even without external impact. Shi et al. 239 obtained non-isocyanate polyurethanes based on BHMF, 1,4-butanediol, and various carbamates. The obtained polymers, being cross-linked with bismaleinimide, also acquire self-healing properties. Thus, BHMF represents a modifying comonomer that can further undergo a reversible Diels – Alder reaction, thus ensuring the ability of the polymer to self-healing upon mechanical damage. The use of such materials can extend the service life of coatings and composite products based on them.
Upare et al. 238 proposed a method for the production of BHMF in butanol, followed by obtaining polyester without isolating BHMF, by treating the reaction solution with succinic acid at 20 °C for 24 h, with 100% conversion of BHMF. The resultant polymer has a glass transition temperature of 99 °C, thermal stability up to 250 °C, and molecular weight of ∼4046 g mol−1 (gel permeation chromatography data).
Oh et al. 240 reported the solvent-free, solid-state mechanochemical synthesis of BHMF-derived polyurethane providing polyurethanes with a molecular weight above 163 000 g mol−1, the glass transition temperature of 96 °C, and thermal stability up to 197 °C (Fig. 21).
Figure 21. Schematic representation of the synthesis of polyurethane in a ball mill using BHMF and MDI (a); screening of polymerization process by controlling the rotation frequency and the reaction time (b); screening of polymerization process by varying catalysts (c); comparison of conversion achieved by using the ball mill with that at the classical synthesis in solution (d). 240 Reprinted with the permission of the American Chemical Society.
Download figure:
Standard imageTo sum up, the most valuable property of the cross-linked polyesters and polyurethanes is their self-healing ability. Also, BHMF has good prospects for application as a comonomer, together with other diols, to provide improved properties to FDCA-based co-polyesters.
4. Processing of by-products of synthesis of furan platform chemicals
Since the development of completely waste-free synthetic methods, especially when using raw materials of plant origin, is a challenge that has not yet been solved, the state-of-the-art techniques for obtaining furan platform chemicals lead to the formation of liquid and solid wastes known as humins. 27,28 Humins are heterogeneous carbon-containing complex oligomers and polymers consisting mainly of furan compounds with many functional hydroxy, keto, and aldehyde groups. The molecular structure of humins is still being specified, 241 since the chemical composition can vary significantly depending on the type of raw material, 242 the concentration of reactants, 243 temperature, 244 the nature of the solvent, 239,242,245 and the processes of humin isolation and subsequent processing.
Elucidation of chemical structure and mechanism of formation of humins under various conditions is necessary to further increase the efficiency of the catalytic conversion of biomass. 243,245–251 Thus, the process of humin formation was studied by the example of decomposition of 11 reference compounds, including carbohydrates and furfural derivatives, in water and various organic solvents as the reaction media. 239,242 All studied carbohydrates could generate solid humins both in water and in organic solvents, except for ethanol, while furfural derivatives could produce solid humins only in water (Fig. 22). These results were explained by the formation of α-ketoaldehydes and α,β-unsaturated aldehydes, acting as primary precursors of humins.
Figure 22. Scheme for the humin formation from the model compounds. 242
Download figure:
Standard imageAs the number of studies concerning the synthesis of HMF in IL has increased in recent years, 77,85,87 Xu et al. 245 attempted to establish the mechanism of humin formation during the conversion of glucose (fructose) into HMF in imidazolium IL ([Bmim]Cl) using CrCl3 as a catalyst. The results showed that it is possible to improve the stability of furan compounds and reduce the amount of humins in IL by converting the hydroxymethyl group into a methoxymethyl one, which increases the solubility of the target products in an organic solvent and facilitates their separation from IL by extraction. 245
As a result of the study of the size evolution, morphology, volume fraction, and concentration of humins formed during dehydration of fructose to give HMF, the competition between the nucleation and growth of humins was discovered (Fig. 23). 243 At a high concentration of HMF, nucleation and growth due to chemical condensation prevail, the number of particles continues to increase. In contrast, at a low concentration of HMF, aggregation and precipitation of humins predominate over chemical condensation, the population decreases, while the particle size increases. Monitoring of the humin growth shows that they are formed mainly from HMF, rather than from fructose.
Figure 23. Scheme for humin formation and growth. Dashed lines show the addition of HMF to other HMF molecules, soluble oligomers, primary particles, or particle aggregates. Solid lines show the process of particle formation, growth, and aggregation. 243
Download figure:
Standard imageShen et al. 244 traced the entire path of humin formation from the feedstock in solution to solid humins. Differences in the structure of intermediates depending on the temperature conditions in the synthesis of HMF were established (Fig. 24).
Figure 24. Plausible variants of the structure of HMF-based humins at a temperature of 4 °C (a), 20 °C (b), and 170 °C (c). 244 Reprinted with the permission of Wiley.
Download figure:
Standard imageThe development of the concept of a circular economy, 50,252,253 aimed at using waste or by-products of biomass processing plants to obtain various value-added goods, in the future will lead to the need for stock-keeping and storage of these wastes on an industrial scale. In this regard, Miralidhara et al. 254 made the first attempt to study the thermal and fire hazards of humins to ensure the conditions for their safe storage, handling, transportation, and processing. The general fire hazard of humins was found to be similar to that of ordinary wood materials.
The amount of formed humins depends on various synthesis parameters and, according to some reports, can reach 30 – 40%. 30 In the case of launching large-scale production of HMF, LA, FDCA, tens of thousands of tons of humins will be obtained as a by-product. Rational processing of such volumes of industrial waste requires the development of simple and effective processes in which humins will be converted into high value-added products.
Until recently, the main methods of utilizing humins included obtaining energy and heat through combustion and gasification. Most of the research was limited to the study of the possibility of using humins as a feedstock for the production of syngas 255 or components of liquid fuels by thermochemical conversion 29,30 or catalytic transformations. 256–261 Pyrolysis of solid humins gave complex multicomponent mixtures containing furans, lactones, phenols, and organic acids. 29,30 The possibility to extract 5-methoxymethylfurfural from humins followed by its hydrogenation (demethoxylation) to 5-methoxymethylfurfuryl alcohol (MTHFA) with 100% conversion was shown. 262
The catalytic selective conversion of humins into cyclic hydrocarbons in high yield can be exemplified by a study, 259 in which Ru/W–P–Si–O bifunctional catalyst was tested for this purpose (Fig. 25). It was shown that Ru nanoparticles additionally catalyze the cleavage of the carbon – carbon bond and the hydrodeoxygenation of small particles (molecules) of the humic polymer, providing the yield of cyclic hydrocarbons of up to 88.3%. In turn, the W–P–Si–O Lewis acid catalyzes the Diels – Alder reaction involving furan rings to afford cycloalkanes. The above-mentioned study demonstrated that biomass conversion can produce not only C5 –C8 compounds but also a wide variety of products with a chain length from C7 to C15.
Figure 25. General scheme for the formation of humins and their catalytic transformation into cyclic hydrocarbons on a bifunctional catalyst Ru/W-P-Si-O. 259 Reprinted with the permission of Elsevier.
Download figure:
Standard imageAnother repidly growing area in the utilization of liquid humins, which provides the production of the value-added products, is their use as a new class of green thermosetting materials. 37–47,263–265 Studies of the kinetics of the processes of auto-cross-linking of humins under the action of temperature are being carried out to obtain thermosetting materials. 263,264 The possibility of obtaining various multicomponent thermosetting materials via co-polymerization of humins with aliphatic bis-epoxides, 37,38 epoxidized linseed oil, 39,40 glycidylated phloroglucinol derived from the biomass phenolic fraction is being investigated. 41
The mechanism for the chain initiation and propagation during the formation of humin copolymers with aliphatic bis-epoxides has been proposed (Fig. 26). 38 The resultant copolymers possess high plasticity and elasticity, which is an important advantage in terms of using humins as industrial construction materials. 37
Figure 26. A plausible mechanism for the chain initiation (a) and propagation (b) during copolymerization of humins with aliphatic bisepoxides. 38 Reprinted with the permission of MDPI.
Download figure:
Standard imageCantarutti et al. 41 demonstrated the ability of humins to be copolymerized with another bio-based epoxide resin (triglycidyl ether of phloroglucinol) through the ether bond formation and proposed the mechanism for anionic polymerization under catalysis with a tertiary amine. It was shown that the resin curing occurs via ether bond formation between humin hydroxyl groups and epoxide moieties of phloroglucinol. Differences were established between humins obtained by industrial and laboratory methods.
Liquid humins can be used to increase the strength of wood and adhesion in the manufacture of composites (plywood, fibreboards), 46,47 and also as a semi-viscous thermosetting matrix for impregnating synthetic and natural fibres. 42–45 In this case, 'green' construction composites are produced, which can find application in construction work, motor and aircraft industry. Utilization of such bio-based materials will not only improve the consumer properties of the products obtained but also increase the proportion of renewable carbon in the final products, which in the near future will affect the final cost of the product.
Sangregorio et al. 47 compared the 'humination' process with more classical furfurylation including impregnation of wood with furfuryl alcohol (FA) and its thermal polymerization inside the wood. Low molecular weight and high functionality of furan monomers contained in the liquid fraction of humins provide good impregnation of wood tissues and high hydrophobicity compared to untreated wood. This study demonstrates for the first time the possibility of using humins as an alternative to FA in wood modification, which will solve the main issues associated with wood for outdoor use through the use of resin that is free of toxic compounds.
The polyfurfuryl alcohol (PFA) and humic resins described by Pin et al. 42 have a sufficiently low viscosity to impregnate cellulose fibres and provide cross-linked composites after final curing. It was shown that the tensile strength of composites based on PFA – humins is twice as high as that of composites PFA – lignin. Incorporation of humins decreases the brittleness of furanic matrix, which is one of the principal disadvantages of the pure PFA composites, and to strenghthen interfacial bonding with cellulose fibres.
The possibility of preparing 'green' composites by compression moulding of flax fibres with humins as a one-component thermosetting matrix has been investigated 43,44 (Fig. 27). Due to the good adhesion between humins and natural fibres, hydrophobic composites could be prepared.
Figure 27. SEM pictures of the tensile fracture surface of humins – flax composites (a); details at a high magnification at the matrix – fibre interface (b, c, d). 44 Reprinted with the permission of Elsevier.
Download figure:
Standard imageIt was shown that new-generation composites based on industrial humins as a thermosetting matrix and jute fibre have low water uptake, good dimensional stability after immersion in water, and therefore, such materials are considered as an alternative to PFA. 45
Kang et al. 266 utilized humins to replace phenol in the production of the humin-phenol – formaldehyde adhesive (HPFA). A two-step process was elaborated including (1) dissolution of humins in alkali through hydrothermal treatment, and (2) polycondensation by replacing 50% of phenol with humins dissolved in alkali. HPFA represents a potential wood adhesive, all six key characteristics of which (dynamic viscosity, bonding strength, pH value, free formaldehyde level, free phenol level, and solids content) meet the national standards of several countries. 266 Also, HPFA has a moderate curing temperature (110 °C), much lower than those of the original (136 °C) and lignin-modified (157 – 188 °C) phenol-formaldehyde adhesives.
Humin-based conductive polymer composites for bipolar plates of low-temperature polymer electrolyte membrane fuel cells (PEMFC) were prepared by a hot-pressing technique. 265 The study of the effect of the humin content (5 – 30 mass %) and the type of conductive filler (natural, oxidized, and colloidal graphite) showed that with a resin content of 10 mass % and the use of colloidal graphite, a balance between electrical conductivity and mechanical properties of the composite is achieved. The interfacial contacting resistance, flexural and compressive strength for this material were 0.035 Ω cm2; 18.4 and 21.4 MPa, respectively.
Liquid humins can also be a basis for obtaining one- 33,267 and two-component 268 foams with controlled morphology and porosity. Tosi et al. 267 developed a protocol, which allows producing of foam-like materials with open and(or) closed cells with a diameter from 0.2 to 3.5 mm. Auto-cross-linking occurs as a result of aldol condensation, rearrangements, and condensation of furan. Humin foams have high thermal stability and low thermal conductivity (lower than polyurethane foams), are versatile materials, economical and easy to manufacture, and can be used as insulating materials and CO2 sorbents. 33 The cellular structure of self-blowing two-component tannin – furanic foams was shown to depend on the foam expansion temperature. 268 Foam expansion at ambient temperature leads to the formation of the closed cells, while at 80 °C, open-cell foams are formed (Fig. 28). Closed-cell foams are suitable for heat insulation materials, while open-cell foams can find use in acoustic insulation and absorption applications.
Figure 28. SEM pictures of humin foams at 300x (a) and 10000x (b) magnification cured at ambient temperature; at 80x (c), and at 800x(d) magnification cured at 80 °C. 268 Reprinted with the permission of MDPI.
Download figure:
Standard imageAnother area of the utilization of solid humins can be their use as a feedstock for the production of porous activated carbon materials used as sorbents for wastewater treatment, 31,32 CO2 sorbents, 33 fillers for the production of special-purpose enamels, 222 and also as electrode materials for electrochemical power industry devices. 34
Solid humins were employed to produce activated carbon during pyrolysis at a temperature in the range of 300 – 700 °C in the presence of phosphoric acid. 31 Pyrolysis temperature was shown to be a key factor affecting pore formation. Resultant activated carbons have high BET surface area of 2375 m2 g−1, BJH pore volume of 0.88 cm3 g−1, and excellent adsorption capacity of 1125 mg g−1, in relation to methylene blue (MB) calculated according to the Langmuir equation.
Chernysheva et al. 34 prepared and tested for the first time activated carbons derived from solid humins as supercapacitor electrode materials (SC). The tests revealed high values of specific capacitance of 370 F g−1 (at a current density of 0.5 A g−1) and excellent stability with retaintion of 92% capacitance after 10 000 charge/discharge cycles (at 10 A g−1 in 6 M KOH). The possibility of the practical application of the obtained material in symmetric two-electrode SC cells as a power source for electrical devices was demonstrated (Fig. 29).
Figure 29. Picture of the power supply for a calculator (a) and a red LED (b). 34 Reprinted with the permission of Wiley.
Download figure:
Standard imageStudies 35,36 demonstrated that humins can be used for the preparation of various heterogeneous catalysts. The common feature of these studies is the implementation of the mechanochemical treatment of humic materials. A study on the possibility of using humins for the production of catalytic nanocomposites with mixed phases of iron oxide possessing magnetic properties was carried out. 35 The composites were prepared by a solvent-free mechanochemical method using a ball mill or by thermal decomposition. It was found that all composites display high catalytic activity in the oxidation of isoeugenol to vanillin under microwave irradiation.
Humins powdered in a ball mill were used for the photodegradation of the Malachite green (MG) dye in an aqueous solution. 36 The photocatalytic activity of humins under visible light irradiation directly depends on the time of mechanical treatment, during which oxygen- and sulfur-containing functional groups are formed on the surface, and the specific surface area of the material also increases (Fig. 30).
Figure 30. Schematic representation of the main steps of the preparation of functionalized humins via ball milling and tests of their photocatalytic activity. 36 Reprinted with the permission of Elsevier.
Download figure:
Standard imageThe potential for preparing anti-corrosive additives 48 or coatings 49 based on liquid humins was explored. Thus, the products of catalytic conversion of biomass (HMF, DFF), as well as the humins formed in this process, were studied as inhibitors of corrosion of low-carbon steel in sulfuric acid in a temperature range of 25 – 90 °C. 48 It was found that all three additives show a moderate protective effect in an acidic medium, with protection degree exceeding 80% in 1 M H2SO4 at room temperature. Humins and DFF behave as cathodic type inhibitors and HMF is a mixed type inhibitor.
Faddeev et al. 49 used humins to prepare anti-corrosive and conductive polymer coating for titanium bipolar plates for low-temperature electrolyte membrane fuel cells (PEMFC). Being dissolved in acetone, subjected to drying and heat treatment (5 min, 180 °C), humins form on a titanium surface a strong and flexible coating with a thickness of 1 μm, resistant to mechanical stress and bending. In addition to corrosive resistance and conductivity, humin-based coatings reduce interfacial contacting resistance of pure titanium to 40%.
The encouraging results give evidence of significant potential for utilization of by-products from the conversion of renewable biomass (solid and liquid humins) to produce various functional materials that can be a worthy substitute to similar petroleum-derived materials. The use of plant biomass will increase the proportion of renewable carbon in final products, which will not only affect the final cost of the product but also reduce the adverse impact of the processing industry on the environment.
5. Economic assessment of the prospects for the use of renewable plant biomass for the production of furanic monomers and polymers
Fossil resources are currently the main source of energy and raw materials for the chemical industry. The negative environmental impact of fossil resources, accompanied by their rapid depletion, forced the scientific community and governments of different countries to look for an alternative source of raw materials. 7 Plant biomass is a renewable feedstock and has great potential for the production of fuels and chemicals due to technological advances and experience gained during the development of fossil resources. Integration of biomass processing into the existing structure of petroleum refineries with the construction of so-called biorefineries can contribute to sustainable development by replacing fossil-based products with bio-based value-added products without the need to develop new infrastructure. The concept of using biomass in refineries can be one of the model systems for the future development of a closed-loop economy (cyclic economy or circular economy). 2,50 Behind the principles of a circular economy (produced-used-reused) (Fig. 31), there are ideas about the use of renewable resources, recycling of secondary raw materials, moving from fossil fuels to the use of renewable energy sources, reducing material flows, and the idea of sustainable consumption, as opposed to an excessive linear economy existing under a slogan take-make-waste. 21,252,253 One main area of the cyclic economy is the development of bio-based chemical compounds in general and plastics, in particular, to facilitate the transition to a low-carbon economy with minimal CO2 emissions into the atmosphere, which is important for climate change mitigating and conserving the Earth's natural resources. 50,51 The interest in this subject is well illustrated by the vast amount of articles and reviews published over the past three to four years, including those in world top journals. Given here are just a few of these publications. 2,21,50,51,252,253,269,270
Figure 31. Circular economy conceptual diagram. 253 Reprinted with the permission of Elsevier.
Download figure:
Standard imageRecently, there has been an increasing concern of the international community about the economic and environmental aspects of the growth in the production and consumption of polymers. 21 This is because, according to some estimates, from 1950 to 2015, 8300 million tons of plastics was produced in the world, of which 6300 million tons were waste. Approximately 800 million tons (12%) of this waste were incinerated, and 600 million tons (9%) were recycled, with only 10% of them being recycled more than once. Roughly 60% (4900 million tons) of all plastics ever produced were thrown away and ended up in landfills or as garbage in the environment. If the current ways of using plastic and handling of waste persist, this will inevitably lead to the accumulation of 12 000 million tons of plastic in the environment by 2050. 21 The bulk of the plastics produced is from packaging, which, due to its long service life, an important consumer property, leads to pollution of the land and the global ocean. 21,193 In addition to efforts aimed at increasing the proportion of recyclable plastics obtained from petroleum products, which is also one of the areas of the circular economy, the search for polymer materials produced from renewable biofeedstock that can replace existing plastics and make their use more environmentally friendly is considered as another area. 271,272 The term 'bioplastics' is often used to describe such materials, but the term bio-based plastics is more preferable. This term refers to plastics that are produced from the feedstock obtained from the processing of renewable biomass. 21,271,272 Biobased plastic can be either biodegradable or non-biodegradable. According to European Bioplastic, total bio-based plastics production capacity will increase to 2.87 million tons in 2025. 273
Meanwhile, converting biomass into desired products requires several complex processes of depolymerization, catalytic conversion, separation, and purification. As repeatedly mentioned, furan derivatives such as HMF, FDCA, DFF, LA, and BHMF, have becomeplatform chemicals, a bridge connecting renewable plant biomass with alternative fuels, chemicals, and materials that can replace similar petroleum-derived products. 1–4,9–20 The use of biomass as a feedstock is considered to be virtually carbon neutral, as the CO2 emissions associated with the production and utilization of biomass-derived products can be compensated by CO2 capture during photosynthesis by plants. 5
Research on technologies for the production of HMF and its derivatives is carried out by the largest chemical companies as well as leading research centres around the world, 274 however, most studies are still carried out for systems based on refined sugars or polysaccharides. The reason for this is that the direct production of HMF from plant biomass or plant waste involves significant challenges because of the low content of easily dehydrated C6-carbohydrates, the presence of various impurities, and the difficulty of isolating HMF from such reaction mixtures and its relative instability. 1
A possible option for improving the competitiveness of renewable plant materials is the conversion of HMF into other more stable multifunctional building blocks [e.g., 5-chloromethylfurfural (CMF), 5-hydroxymethyl-2-furancarboxylic acid (HMFCA), 1-hydroxyhexane-2,5-dione (HHD), etc.] to produce bio-derivatives of analogues of known products of fine chemical synthesis, including solvents, monomers, pharmaceuticals, and other valuable products, and also biofuels (Fig. 32). 4,275
Figure 32. Various approaches to HMF conversion: a traditional direct derivatization giving FDCA, FDME, FDEE, DFF, LA, DMF, EMF, BHMF, BAMF, GVL (a); conversion of HMF into multifunctional stable building blocks: HMFCA, CMF, HHD, HBO (b). 4 Reprinted with the permission of Wiley.
Download figure:
Standard imageAs a result of a technical and economic assessment of various methods for obtaining HMF and its derivatives based on purified fructose, it was shown that two aspects are important to achieve competitive prices for chemical products based on renewable plant biomass. First, a large amount of raw material for fructose production at reasonable prices must be available close to the production site to minimize transport costs. Second, the energy prices for the production process must be relatively low, since the cost of energy is one of the main factors affecting the production of HMF and other furan compounds. 63 Moreover, the yields of target products, the cost of associated and by-products (for example, LA, humins), catalytic systems, reagents, solvents, and equipment are of great importance. 1 If these criteria are met, large-scale production of platform chemicals will become economically viable, which will provide the growth of the market for bio-based chemicals and help to reduce society's dependence on fossil resources. 63
6. Conclusion
Polymer chemistry today is one of the most actively developing, interesting, and demanded areas both in the field of fundamental research and in solving applied problems. Polymers and composites based on them have a set of consumer properties that make them indispensable in industry and everyday life. However, their production from fossil hydrocarbons and poorly developed plastic recycling technologies are already causing serious environmental damage. Obtaining polymers from biomass, primarily biodegradable, can solve many environmental issues, such as disturbed carbon balance and the associated greenhouse effect, pollution of cities and landscapes, as well as the global ocean with plastic waste.
Plant biomass contains an almost inexhaustible supply of annually renewable carbon, and it is this biomass that can and should become the basis for the production of platform chemicals, monomers and polymers, which will ensure a zero carbon balance, reduce greenhouse gas emissions and reduce environmental impact on nature. Of particular relevance is the utilization of crop waste, which are currently used ineffectively and, along with polymer waste, are an important source of environmental pollution, in chemical processing to obtain valuable chemical compounds and materials. At the same time, this problem could be solved by the development of new processes for the complex processing of plant waste based on carbohydrates and lignocellulose into organic molecules for various applications. It should be noted that the list of reactions involving key intermediates of biomass conversion, platform chemicals intended to ensure the transition to a sustainable chemical industry that uses inedible types of plant raw materials instead of petroleum ones, has been constantly expanding in recent years.
These strategies can be successfully implemented by constructing enterprises for processing plant biomass, operating on the principle of oil refining and petrochemical industries, combining the production of several products in one process flow diagram. These enterprises, by analogy with oil refineries, are called biorefineries, with catalytic technologies playing a decisive role in the success of such an integrated approach. In many developed countries, biorefinery technologies are considered as one of the most effective ways to address environmental, energy, and raw material problems. Complex processing of biomass also implies the disposal of the resulting waste, since completely waste-free synthesis methods, especially when using raw materials of plant origin, are difficult to implement. Conversion of solid and liquid waste such as humins into useful products (porous activated carbon materials, sorbents, electrode materials, heterogeneous catalysts, composites, etc.) is an essential challenge. This approach meets modern requirements for developing a circular economy.
Despite the significant progress achieved in recent years in the conversion of biomass and carbohydrates into HMF, FDCA, DFF, BHMF, LA, other furan compounds, monomers, and polymers, most of the proposed synthetic approaches and production processes are still characterized by low efficiency and high cost of end products as compared to similar processes and compounds derived from fossil raw materials. For a long time, the efforts of the scientific community and large chemical companies (Merck, BASF, Archer Daniels Midland Company, Atlas Ind., Avantium, Avalon, Brasket, R&F Chemical, etc.) have been aimed at realizing the industrial production of HMF and other platform chemicals, as well as polymers based on them. However, the large-scale production of these materials remains unimplemented. This indicates the need to consolidate efforts and continue work to find innovative approaches and highly efficient catalytic systems for converting biomass into high value-added products. Also, an objective assessment of the efficiency, economic and practical feasibility, as well as the environmental safety of the developed processes and technologies is required.
This review was financially supported by the Russian Science Foundation (Project No. 21-13-00177).
7. List of acronyms
| AAc | —acrylic acid; |
| ABS | —acrylonitrile butadiene styrene; |
| 4-AcNH-TEMPO | —4-acetylamino-2,2,6,6-tetramethylpiperidine-1-oxyl; |
| BAMF | —2,5-bis(aminomethyl)furan; |
| BmimCl | —1-n-butyl-3-methylimidazolium chloride; |
| BHEFDC | —diethylene glycol 2,5-furandicarboxylate; |
| BHMF | —2,5-bis(hydroxymethyl)furan; |
| BHMTHF | —bis(hydroxymethyl)tetrahydrofuran; |
| BPADA | —2,2-bis[4-(3,4-dicarboxyphenoxy)phenyl]-propane dianhydride; |
| BPDA | —3,3',4,4'-biphenyltetracarboxylic acid dianhydride; |
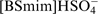
| —1-(4-sulfonic acid)-butyl-3-methylimidazolium hydrogen sulfate; |
| CAP-1 | —N-(3-chloro-4-methylphenyl)-N'-2-[(5-[(dimethylamino)methyl]-2-furylmethyl)sulfanyl]ethylurea; |
| CL | —ε-caprolactone; |
| CMF | —5-chloromethylfurfural; |
| DADF | —5,5-oxy-bis(methylene)bis-furan-2- carboxylic acid (OBFA) diallyl ester; |
| DAF | —furan-2,5-dicarboxylic acid (FDCA) diallyl ester; |
| DCF | —FDCA dicinnamyl ester; |
| DFF | —2,5-diformylfuran; |
| DMAc | —N,N-dimethylacetamide; |
| DMF | —2,5-dimethylfuran; |
| DMI | —dimethyl itaconate; |
| DMTHF | —2,5-dimethyltetrahydrofuran; |
| DPA | —diphenolic acid; |
| ECO | —electrochemical oxidation; |
| FA | —furfuryl alcohol; |
| 6FDA | —4,4'-4,4'-(hexafluoroisopropylidene)diphthalic anhydride; |
| FDCA | —furan-2,5-dicarboxylic acid; |
| FDCDCl | —furan-2,5-dicarboxylic acid dichloride; |
| m-FDDA | —N,N'-bis(3-aminophenyl)furan-2,5-dicarboxamide; |
| p-FDDA | —N,N'-bis(4-aminophenyl)furan-2,5-dicarboxamide; |
| FDME | —2,5-dimethyl furanoate; |
| FFCA | —5-formylfuran-2-carboxylic acid; |
| g-C3N4 | —graphitic carbon nitride; |
| GVL | —γ-valerolactone; |
| HDO | —1,6-hexanediol; |
| HHD | —1-hydroxyhexane-2,5-dione; |
| HMF | —5-hydroxymethylfurfural; |
| HMFCA | —5-hydroxymethylfuran-2-carboxylic acid; |
| [Hmim]HSO4 | —1-hexyl-3-methylimidazolium hydrogen sulfate; |
| HPFA | —humin – phenol – formaldehyde adhesive; |
| IL | —ionic liquid; |
| KVL | —4-ketovalerolactone; |
| LA | —levulinic acid; |
| MA | —maleic anhydride; |
| MAAc | —methacrylic acid; |
| MDI | —methylenediphenyl diisocyanate; |
| MIBK | —methyl isobutyl ketone; |
| NMP | —N-methylpyrrolidone; |
| OBFA | —5,5-oxy-bis(methylene)bis-furan-2- carboxylic acid; |
| PAI | —polyamidimide; |
| PA-6 | —polyamide-6; |
| PA8F | —poly(octamethylene furanamide); |
| PBF | —poly(butylene furanoate); |
| PBT | —poly(butylene terephthalate); |
| PC | —polycarbonate; |
| PCHDMF | —poly(1,4-cyclohexanedimethylene 2,5-furandicarboxylate); |
| PCN | —unmodified polymeric carbon nitride; |
| PCN-H2O2 | —modified polymeric carbon nitride; |
| PEF | —poly(ethylene furanoate); |
| PEN | —poly(ethylene 2,6-naphthalate); |
| PET | —polyethylene terephthalate; |
| PET-G | —polyethylene terephthalate glycol; |
| PFA | —polyfurfuryl alcohol resins; |
| PHeCF | —poly(hexamethylene 2,5-furandicarboxylateco-caprolactone); |
| PHF | —poly(hexamethylene 2,5-furandicarboxylate); |
| PI | —polyimide; |
| PLA | —polylactide; |
| PMoV HPA | —vanadium-substituted heteropolyacids; |
| PPeCF | —poly(pentylene 2,5-furandicarboxylate-co-caprolactone); |
| PPF | —poly(propylene furanoate); |
| RHE | —reversible hydrogen electrode; |
| TEMPO | —2,2,6,6-tetramethylpiperidine-1-oxyl. |